Catch me if you can—How to make the best use of pleural effusion
Introduction
Multiple myeloma (MM), one the most common malignant hematological disease, accounts for 1% of all malignant neoplasm and 10% of hematological malignancy. About 6% of patients with MM may have pleural effusion. However, only 0.8% to 2.6% of patients with MM have myelomatous pleural effusion (MPE) (1,2). The diagnosis of MPE includes detection of atypical plasma cells in pleural fluid, demonstration of monoclonal protein in pleural fluid electrophoresis or histologic confirmation using pleural biopsy specimen or autopsy (3). MPE is regarded as a late stage of MM with poor prognosis and the median survival time is about 4 months (1).
The rarity of MPE let the clinicians in dilemma. Although challenging, it is of vital importance to diagnose MPE as early as possible. We report an extremely rare case of MM [IgD-lambda (λ) type, stage III B] with MPE as initial presentation and complicated with massive pericardial effusions during the progression of the disease. What we learn from this case is how to make the best use of pleural effusion.
Case presentation
A 72-year-old man presented for chest pain and dyspnea for four months. He felt paroxysmal chest pain, which was aggravated with severe cough. He had exertion associated dyspnea for one month. His medical history included atrial fibrillation, sick sinus syndrome, and implantation of artificial permanent pacemaker. Physical examination revealed the dullness to percussion and the obvious decrease in audible breath sounds in left lower chest wall.
Blood investigation revealed the following results—white blood cell (WBC): 4.40×109/L (normal range: 3.5×109–9.5×109/L); neutrophils: 70% (normal range: 50–70%); hemoglobin: 110 g/L (normal range: 137–179 g/L); platelet count: 232×109/L (normal range: 100×109–300×109/L); S-CRP: 14.32 mg/L (normal range: 0–3 mg/L); erythrocyte sedimentation rate (ESR): 65 mm/hr (normal range: 0–15 mm/hr); total protein: 79.3 g/L (normal range: 60–82 g/L); albumin: 38 g/L (normal range: 35–50 g/L); blood calcium: 2.33 mmol/L (normal range: 2.12–2.75 mmol/L); uric acid: 502 µmol/L (normal range: 150–420 µmol/L); creatine: 116.60 µmol/L (normal range: 44–133 µmol/L); BUN: 6.54 mmol/L (normal range: 1.8–7.1 mmol/L); ALT and AST was normal; LDH: 258 IU/L (normal range: 100–240 IU/L); HBDH: 252 IU/L (normal range: 90–220 IU/L); ABG: pH 7.47, PO2 82 mmHg, PCO2 33 mmHg; BNP: 323.70 pg/mL (normal range: <100 pg/mL); Pro-GRP: 39.57 pg/mL (normal range: <22.0 pg/mL) and other serum tumor markers were within normal range. T-SPOT.TB was negative.
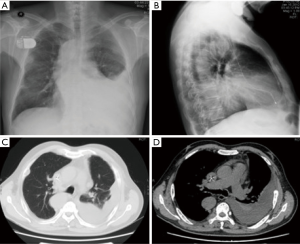
Chest radiograph (Figure 1) showed left pleural effusion and the computed tomographic image of the chest (Figure 1) showed left massive pleural effusion, pulmonary atelectasis of left lower lobe, and multiple destruction of bones in bilateral costal bones, thoracic vertebra and left scapula. To detect the origin of malignancy, PET-CT showed multiple abnormally high glucose metabolic sites in bones, ossification, osteolysis and pathologic fracture, left pleural effusion and pulmonary atelectasis of left lower lobe.
To improve dyspnea and detect the cause of pleural effusion, thoracentesis and aspiration of pleural effusion was done.
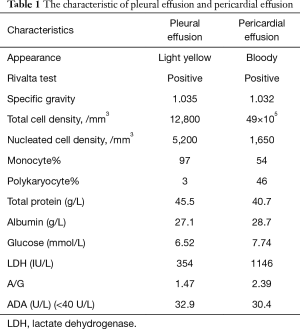
The detailed information of pleural fluid was in Table 1. No bacterium, acid-resistant bacilli or fungus was found. Further smear cytopathology of pleural effusion showed no evidence of malignant cells which was repeated for three times.
Full table
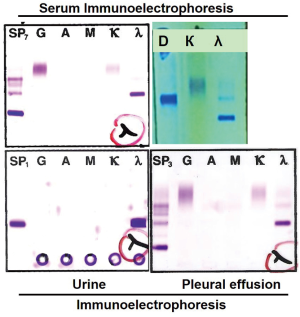
The auxiliary tests to explicit the reasons of bone destruction focused on possibility of the malignant metastatic to bone, or primary malignancy in bone. Immunofixation electrophoresis discovered the existence of monocolonal IgD λ light chain in serum and urine. The concentration of light chain in serum, urine and pleural effusion was above the normal range. β-2 microglobulin was much higher than the detectable upper limit 10 µg/mL (normal range <2.3 µg/mL) (Figure 2).
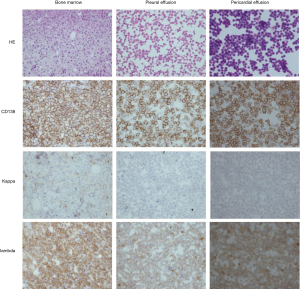
Bone marrow aspiration was taken. Smear showed no abnormal findings. Bone marrow aspiration biopsy confirmed the diagnosis of plasma cell myeloma. Massive hyperplastic, infiltrative round cells showed the characteristic morphologic features of abnormal plasma cells, with eccentric nucleus. Immunohistochemistry staining showed that those cells were positively stained for CD138 (+++) and λ (+++), and negatively for κ (Figure 3). The Ki67 was 50–70%. Flow cytometry confirmed the existence of myelomatous cells.
The diagnosis of MM [IgD-lambda (λ) type, stage III B] with MPE was confirmed. However, what is the relationship between pleural effusion and MM, after negative results of pleural effusion smear?
The pleural effusion increased rapidly and it was urgent to clarify the reason of pleural effusion. The smear of pleural effusion showed no evidence of malignancy after repeated cytology smear. To capture the possible cancer cells in pleural effusion, pleural effusions were centrifuged and the sedimentation was embedded with wax to make cell block for immunocytochemistry. It was exciting and amazing to discover the myelomatous cells expressing CD138 (++) and λ (++) in pleural effusion (Figure 3). Moreover, protein electrophoresis of pleural effusion showed M protein and immunofixation electrophoresis demonstrated the existence of monoclonal λ band. And the concentration of λ in pleural effusion was high, similar to that in serum. Flow cytometry confirmed the existence of myelomatous cells in pleural effusion which were the same as those in bone marrow.
The diagnosis of MM [IgD-lambda (λ) type, stage III B] with MPE was confirmed. After the false tale told by pleural effusion smear, cell block of pleural effusion uncovered the real reason. The protein electrophoresis, immunofixation electrophoresis, concentration of light chain and flow cytometry of pleural effusion enriched our understanding about the rare complication of MM.
After one cycle of chemotherapy, the pleural effusion improved and only a small volume of pleural effusion was left, confirmed by chest X-ray. Unfortunately, four months later, the patient was admitted to hospital emergently due to dyspnea. Further examination demonstrated respiratory failure, massive pericardial effusion and pericardial tamponade. About 1,400 mL pericardial effusion was aspirated intermittently. The detailed information of pericardial effusion was in Table 1. The myelomatous cells were observed in the pericardial effusion confirmed by immunohistopathology (Figure 3), and flow cytometry. Invasion of MM to pericardium was diagnosed and the patient accepted BD chemotherapy (bortezomib and dexamethasone). Dyspnea was improved and the following UCG showed a small amount of pericardial effusion. However, with the disease progression, the patient died of multiple organ dysfunction ten months later.
Discussion
MM is one the most common malignant hematological disease which accounts for 1% of all malignant neoplasm and 10% of hematological malignancy. Pleural effusion exists in about 6% of patients with MM, while MPE is rarer, and accounts for only 0.8% to 2.6% of pleural effusions in patients with MM (1,2). Usually, pleural effusion is due to many other etiologies, such as congestive heart failure, renal failure, nephrotic syndrome, pulmonary thromboembolism, amyloidosis, infection, and second malignancy (2,3). The diagnosis of MPE includes detection of atypical plasma cells in pleural fluid, demonstration of monoclonal protein in pleural fluid electrophoresis or histologic confirmation using pleural biopsy specimen or autopsy (3). Myelomatous involvement of pleura or adjacent tissues were the most common reasons of MPE. MPE is regarded as a late stage of MM with poor prognosis and the median survival time is about 4 months (1).
In published literature, 80% of MPE cases were due to IgA subtype, followed by IgG subtype. MPE as an initial sign of MM is extremely rare and only 16 cases were reported in English literature since 2000 according to Zhang et al. (4). Myelomatous pericardial effusion has been reported sporadically but this is a much rarer complication compared with MPE (5). With these informations above, we can conclude that this case reported here is extremely rare.
The ADA activity in the case reported here was less than 40 IU/L, however, it was noteworthy to mention the high ADA activity in some MPEs. High ADA activity usually favors tuberculous pleural effusion. It is interesting to find high ADA activity in patients with MPE who had no evidence of active pulmonary tuberculosis (6). ADA is expressed in activated T-lymphocytes, so the elevation of ADA activity in pleural effusion may be an indicator of active local inflammatory response.
Due to the rarity of MPE, the clinical dilemma may lead to misdiagnosis or delayed diagnosis. The preferred methods for diagnosis of MPE include pleural fluid cytology or pleural biopsy. First of all, cytological identification of malignant plasma cells in pleural effusion is the most direct method for diagnosis. However, the limited number of malignant plasma cells in pleural effusion and in vitro cellular destruction or degeneration may lead to false diagnosis. Secondly, if there are abnormal signs of pleural in CT images or B-ultrasonography, CT/B-ultrasound guided pleural biopsy is likely to get positive result. However, pleural biopsy guided with CT/B-ultrasound may fail to confirm the diagnosis probably due to the discontinuous myelomatous affectation of the pleura. It is reported that the diagnostic value of thoracoscopy has been associated with a higher positive rate of diagnosis of malignant pleural effusion. However, if pleural fluid can be fully investigated, the invasive thoracoscopy may be avoided. So, how to make good use of pleural effusion is a challenging but essential problem.
Cytological identification and confirmation of malignant plasma cells in pleural effusion is indispensable for the diagnosis. The commonly available methods include cell smear and cell block. The tale told by this case showed that cell block supplied more information than cell smear. Several studies compared the conventional cytology smear and the cell block technique and concluded that cell block can increase the sensitivity of detecting malignancies, with increased cellularity, better morphological details and available immunohistochemistry staining (7). Cell block is superior to smear technique in staging of tumor and rapid identification.
In conclusion, we report the extremely rare case of IgD-lambda MM presented as MPE initially and complicated with massive pericardial effusion later. Moreover, it is very important to popularize the clinical application of cell block technique, to acquire better diagnosis and avoid unnecessary thoracoscopy.
Acknowledgements
Funding: This study was supported by the grants from the National Natural Science Foundation of China (grant number 30971313, 81141001, 81270114 and 81670043).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma 2005;46:1137-42. [Crossref] [PubMed]
- Kintzer JS Jr, Rosenow EC 3rd, Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma. A review of 958 cases. Arch Intern Med 1978;138:727-30. [Crossref] [PubMed]
- Oudart JB, Maquart FX, Semouma O, et al. Pleural effusion in a patient with multiple myeloma. Clin Chem 2012;58:672-4. [Crossref] [PubMed]
- Zhang LL, Li YY, Hu CP, et al. Myelomatous pleural effusion as an initial sign of multiple myeloma-a case report and review of literature. J Thorac Dis 2014;6:E152-9. [PubMed]
- Abelman W, Virchis A, Yong K. Extramedullary myeloma representing as a pericardial effusion with tamponade: two case reports and a further review of 19 cases in the literature. Leuk Lymphoma 2005;46:137-42. [Crossref] [PubMed]
- Cho YU, Chi HS, Park CJ, et al. Myelomatous Pleural Effusion: A Case Series in a Single Institution and Literature Review. Korean J Lab Med 2011;31:225-30. [Crossref] [PubMed]
- Ugurluoglu C, Kurtipek E, Unlu Y, et al. Importance of the cell block technique in diagnosing patients with non-small cell carcinoma accompanied by pleural effusion. Asian Pac J Cancer Prev 2015;16:3057-60. [Crossref] [PubMed]