The clinical usefulness of a self-administered questionnaire for sleep-disordered breathing in patients with neuromuscular disease
Introduction
The term neuromuscular disease (NMD) summarises a broad spectrum of acquired and inherited conditions which range in disease severity (1). Pathological processes underlying NMD vary depending on disease aetiology, they can affect muscle structure and function, such as in myotonic dystrophy (MD), or the neuromuscular junction, such as in myasthenia gravis (MG). In amyotrophic lateral sclerosis (ALS), NMD can also cause dysfunction of the efferent nerve (2), whilst the central nervous system (CNS) can be involved, as in stroke. In NMD patients who develop diaphragm weakness or paralysis, sleep-disordered breathing (SDB) is a complication which contributes significantly to morbidity and mortality (1,3,4). Indeed, undetected SDB can eventually progress to hypercapnic respiratory failure and death (2).
Daytime fatigue and tiredness are cardinal symptoms associated with sleep disturbance (2). Questionnaires like the Epworth Sleepiness Scale (ESS) have been developed to screen for patients with obstructive sleep apnoea (OSA) and associated daytime symptoms (5). However, the ESS has not been designed to identify patients with SDB due to underlying NMD. In particular, individuals in early stages of SDB due to NMD might be breathless and not sleepy and, therefore, may not be reliably identified by the ESS, although available treatment with non-invasive ventilation (NIV) could provide an early benefit (6,7).
To establish the diagnosis of SDB in NMD patients, polysomnography (PSG) and arterial blood gas analysis provide the most reliable method. Indeed, PSG is currently considered as the “gold standard” to diagnose SDB (1,4,8-12). However, the sophisticated setup, the required expertise and the associated costs limit the availability of PSG facilities for adult (13,14) and paediatric (12) patients. Consequently, there is a clinical need to screen for patients with NMD at risk of developing SDB due to respiratory muscle weakness using other available tools and to triage patients at risk for further investigation using PSG. For this purpose, a questionnaire, the ‘SDB in Patients with NMD’ questionnaire (SiNQ-5), has previously been developed and validated using PSG and invasive tests of respiratory muscle strength in patients with NMD and SDB (10).
Following the successful validation of the SiNQ-5 in identifying NMD patients with untreated SDB compared to SDB and healthy subjects, we hypothesised that the SiNQ-5 scores of patients with NMD and treated SDB, would be similar to the scores of NMD patients without SDB and those with OSA, chronic obstructive pulmonary disease (COPD), heart failure (HF) and patients with non-respiratory sleep disorders.
Methods
Consecutive patients from respiratory outpatient settings, the Lane Fox Unit, and the sleep services within Guy’s and St Thomas’ NHS Foundation Trust, London, UK were selected during a 1-year period (01/2011–01/2012). The study was registered as a clinical service evaluation with the local clinical governance committee (registration number with local board: 2012-2979); informed, verbal consent was obtained from all participants. All patient details were anonymised and treated with confidentiality. Based on pre-existing diagnoses, patients were categorised into the following subgroups: NMD with and without SDB, OSA, COPD, HF, and patients with other conditions, which included individuals with non-respiratory sleep disorders (such as narcolepsy, insomnia and parasomnia) and snorers without SDB. Patients with COPD and HF were stable and at optimised treatment. Clinical information regarding treatment with continuous positive airway pressure (CPAP) or NIV, along with the presence or absence of SDB were collected. A diagnosis of SDB was made based on the results of the overnight sleep study results; an apnoea hypopnoea index (AHI) or an oxygen desaturation index (4% ODI) >5/h, or a total sleep time with SpO2 <90% of more than 10% of the night were defined as sleep disordered breathing (8,15).
SiNQ-5
A printed copy of the self-administered 5-item SiNQ-5, each item to be scored between 0 and 2 (total range 0–10 points, a score >5 points indicating increased likelihood of associated SDB) was given to each patient to complete in their own time (10). Responses to the SiNQ-5 were not influenced by clinicians or family members; an exception to this was made when patients experienced problems understanding the content of the questionnaire and required further clarification.
ESS
A subgroup of patients completed the ESS, a self-administered questionnaire designed to measure daytime sleepiness in eight common situations (5). Each item of the ESS was scored on a scale from 0 to 3, allowing for a total score of 0 to 24 points; a score ≥10 points identified patients with excessive daytime sleepiness. The questionnaire had originally been developed and validated for screening of daytime sleepiness in patients with OSA (5), but is also widely used to screen for other conditions causing excessive sleepiness in the general population (16).
Statistical analysis
Data were collected, tabulated and analysed using MS Excel 2016 (Microsoft Corporation, Seattle/WA, USA) and IBM SPSS statistics version 23 (IBM, New York/NY, USA); they are presented as mean (standard deviation), unless stated otherwise. Categorical variables, such as gender, were presented as percentages. The results of the Kolmogornov-Smirnoff test for normality supported the use of non-parametric tests for further analysis; the Mann-Whitney U independent t-test was used to compare different SiNQ-5 scores of NMD patients with and without SDB. Fisher’s exact test was used to investigate whether proportions of groups were different. Kruskal-Wallis one-way-analysis-of-variance was used to identify differences in item-specific and composite scores of the SiNQ-5 between more than two patient groups; Dunn’s and Bonferroni post-hoc correction for multiple testing was applied when Kruskal-Wallis one-way-analysis-of-variance was found to be statistically significant. A Box-Whisker plot and a clustered bar graph were created to demonstrate item-specific SiNQ-5 responses. Spearman’s rank correlation coefficient (rs) was used to assess correlations between SiNQ-5 and ESS responses; this was presented graphically as a scatterplot. P value <0.05 was considered statistically significant.
Results
A total of 298 patients participated, of which data from 33 individuals were excluded based on incomplete datasets, to result in a cohort size of 265 patients. This cohort consisted of 51 patients with NMD (40 of which had SDB), 120 patients with OSA, 16 patients with COPD (14 of which had SDB), 9 patients with HF (7 with SDB), and 69 patients with other conditions. There were differences in the age distribution of the different groups; in particular, patients with non-respiratory disorders were significantly younger than patients with NMD. All but the non-respiratory disorder group contained more male than female subjects (Table 1).

Full table
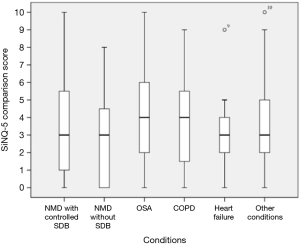
All patients with NMD and SDB were controlled with NIV. There were no differences in SiNQ-5 scores between NMD patients with SDB who were established on NIV treatment and NMD patients without SDB (P=0.417). About 1/3 of NMD patients with SDB on treatment, and just more than 1/4 of NMD patients without SDB scored within the higher score bracket of 5–10 points (P=1.000). Approximately 3/4 of patients with other conditions scored within the lower bracket [0–4], which was not different to NMD patients with treatment controlled SDB (P=0.663); there were no relevant differences between NMD established on treatment and OSA patients (P=0.192) (Table 2).

Full table
Comparison of the SiNQ-5 scores between the subgroups did not show any statistically significant differences (P=0.077); patients with NMD and SDB who were established on NIV scored similar to patients with NMD who had not developed SDB (Figure 1).
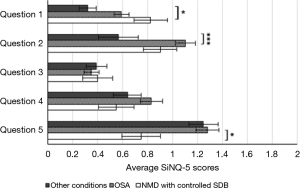
Comparing item-specific responses of all six groups of patients demonstrated significant differences between three groups, NMD patients with SDB, OSA and patients with other conditions. There were differences in item #1, item #2 and item #5 with a difference between NMD patients with controlled SDB and patients with other conditions (item #1), between patients with OSA and those with other conditions (item #2), and between NMD patients and patients with OSA (item #5; Figure 2).
Out of the total cohort, 148 patients completed the ESS. These were patients with OSA (n=78), patients with other conditions (n=60), and patients with NMD (n=10). In OSA patients, a positive correlation was found between ESS and SiNQ-5 scores (r=0.472; P<0.001), but there were no significant correlations in NMD patients (P=0.067), and in patients with other conditions (P=0.810).
Discussion
Patients with neuromuscular conditions who develop SDB score similar on the SiNQ-5 compared to NMD patients without SDB once they are successfully established on NIV. SiNQ-5 scores of patients with NMD compared to patients with OSA and other conditions associated with breathlessness did not identify significant differences. Analysis of SiNQ-5 scores by individual questions revealed that particularly item #1 identified patients with NMD conditions. This item is focused on the development of postural breathlessness. Item #2 assessed whether patients felt breathless when bending forward, such as the motion required in tying shoelaces, and patients with OSA who were more obese than the other subgroups were identified by this question. An association between the ESS and the SiNQ-5 scores potentially indicates that sleep disruption caused daytime symptoms, but this was only shown in the sleep apnoea cohort. These findings add understanding to previous work by the group, which demonstrated accuracy of the SiNQ-5 in identifying patients with NMD at risk of developing SDB (10).
Clinical relevance
A change from the seated to the supine posture has previously been described to result in a reduction of the functional residual capacity (FRC) (17,18). However, a decrease in the operating lung volume, combined with a shift of the abdominal contents with gravity impacting on a weak diaphragm can further contribute to worsening respiratory function when supine (19,20). Due to the obesity epidemic (21-23), OSA is the most common respiratory sleep disorder (17). Various questionnaires exist to screen patients for patients with OSA (2), the most common examples include the Berlin Questionnaire, which screens for sleep apnoea in the primary care setting (24), the ESS (5), and the STOP-BANG questionnaire, which specifically screens for OSA (25,26). In contrast to sleep apnoea, there are scarce tools to screen for respiratory sleep disorders in NMD. However, the existing clinical need for a questionnaire in NMD has led to the development of the SiNQ-5 (10).
Screening for SDB in NMD can be challenging owing to the wide spectrum of conditions (1,2). However, SDB has been highlighted as a common feature of patients with a weak diaphragm (20). Convincingly, management of SDB in NMD with NIV leads to significant improvements in the AHI, oxygenation, hypoventilation and symptom control, improving quality of life and reducing disease-related mortality (7,27,28). Hence, early detection and treatment of the condition is important. Indeed, in progressive NMD like motor neuron disease (MND), unmanaged SDB can result in respiratory failure and death (27).
The SiNQ-5 may be useful in guiding clinical decision making, but it is no adequate replacement for PSG studies. However, a high score in the SiNQ-5 (5–10 points) is a risk indicator for SDB in NMD patients, and can help to support the decision to refer for PSG or to guide timely intervention. In this context, the SiNQ-5 should be used alongside standard lung function tests and careful history taking (1,2,27), as well as arterial blood gas analysis.
Notably, no significant differences were found between NMD patients with controlled SDB and NMD patients without SDB. This finding may be interpreted as evidence that once controlled with NIV, symptoms of SDB were alleviated to the extent that no differences could be found when compared to NMD patients without respiratory muscle involvement. The varied nature of different NMD requires further investigation to determine whether the SiNQ-5 could be used to monitor disease progression or improvements during setup of NIV.
Limitations of the study
Patients in the current study cohort were included from clinic visits in the outpatient settings and PSG services. This method of sampling offered a realistic snapshot of questionnaire responses in a standard clinical setting. However, this will also have led to unequal sample sizes in different subgroups and selection bias. Furthermore, NMD patients with SDB were already established on NIV. Ideally, an experiment design would select for equal numbers of NMD patients with and without SDB and assess patients prior to and following setup on NIV to see whether the SiNQ-5 score is responsive to a change in the clinical circumstances. NMD patients with controlled SDB were determined as such with sleep studies or overnight pulse oximetry on therapy, and a review of whether SDB had been controlled or was still present. However, variations in the degree of control and treatment adherence, as well as in the patients’ perceived symptomatic improvement could have confounded the SiNQ-5 scores. Quantitative measures of the degree of control obtained in a sleep laboratory setting would have been useful in addressing these limitations.
Conclusions
Patients with NMD and SDB who are well controlled with NIV cannot be identified by means of the SiNQ-5. Further studies are necessary to further explore the responsiveness of the questionnaire when clinically monitoring patients with newly diagnosed NMD and SDB prior to commencing on NIV.
Acknowledgements
Dr. J Steier’s contribution to this work was partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was registered as a clinical service evaluation with the local clinical governance committee (registration number with local board: 2012-2979); informed, verbal consent was obtained from all participants.
References
- Aboussouan LS. Sleep-disordered Breathing in Neuromuscular Disease. Am J Respir Crit Care Med 2015;191:979-89. [Crossref] [PubMed]
- Cao M, George C, Guilleminault C. Sleep and Neuromuscular Diseases. In: Kryger MH, Roth T, William CD, editors. Principles and Practice of Sleep Medicine. 5th ed. Canada: Saunders, 2010:1015-25.
- Ambrosino N, Carpenè N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J 2009;34:444-51. [Crossref] [PubMed]
- Jennum P, Santamaria Cano J, Bassetti C, et al. Sleep disorders in neurodegenerative disorders and stroke. In: Gilhus N, Barnes M, Brainin M, editors. European handbook of neurological management. 2nd ed. Singapore: Blackwell Publishing Ltd., 2011:529-43.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Lofaso F, Fauroux B, Orlikowski D, et al. Daytime predictors of sleep-disordered breathing in neuromuscular patients to better schedule polysomnography. Eur Respir J 2011;37:231-2. [Crossref] [PubMed]
- Bourke SC, Gibson GJ. Sleep and breathing in neuromuscular disease. Eur Respir J 2002;19:1194-201. [Crossref] [PubMed]
- Fleetham J, Ayas N, Bradley D, et al. Canadian Thoracic Society guidelines : Diagnosis and treatment of sleep disordered breathing in adults. Can Respir J 2006;13:387-92. [Crossref] [PubMed]
- Kushida C, Littner M, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 2005;28:499-521. [Crossref] [PubMed]
- Steier J, Jolley CJ, Seymour J, et al. Screening for sleep-disordered breathing in neuromuscular disease using a questionnaire for symptoms associated with diaphragm paralysis. Eur Respir J 2011;37:400-5. [Crossref] [PubMed]
- Chokroverty S. Sleep-disordered breathing in neuromuscular disorders: a condition in search of recognition. Muscle Nerve 2001;24:451-5. [Crossref] [PubMed]
- Burke RM, Maxwell B, Hunter C, et al. Night-to-night variation of pulse oximetry in children with sleep-disordered breathing. Arch Dis Child 2016;101:1095-9. [Crossref] [PubMed]
- Flemons WW, Douglas NJ, Kuna ST, et al. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med 2004;169:668-72. [Crossref] [PubMed]
- Steier J, Martin A, Harris J, et al. Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax 2014;69:390-2. [Crossref] [PubMed]
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical Guidelines for the Evaluation, Management and Long - term care of Obstructive Sleep Apnea in Adults. J Clin Sleep Med 2009;5:263-76. [PubMed]
- Boyes J, Drakatos P, Jarrold I, et al. The use of an online Epworth Sleepiness Scale to assess excessive daytime sleepiness. Sleep Breath 2017;21:333-40. [Crossref] [PubMed]
- Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014;69:752-9. [Crossref] [PubMed]
- Ibañez J, Raurich J. Normal values of functional residual capacity in the sitting and supine positions. Intensive Care Med 1982;8:173-7. [Crossref] [PubMed]
- Dean E. Effect of body position on pulmonary function. Phys Ther 1985;65:613-8. [Crossref] [PubMed]
- Steier J, Jolley CJ, Seymour J, et al. Sleep-disordered breathing in unilateral diaphragm paralysis or severe weakness. Eur Respir J 2008;32:1479-87. [Crossref] [PubMed]
- Jou C. The biology and genetics of obesity - a century of inquiries. N Engl J Med 2014;370:1874-7. [Crossref] [PubMed]
- Sharma AM. M, M, M & M: A mnemonic for assessing obesity. Obes Rev 2010;11:808-9. [Crossref] [PubMed]
- Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. Interactions Between Obesity and Obstructive Sleep Apnea. Chest 2010;137:711-9. [Crossref] [PubMed]
- Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485-91. [Crossref] [PubMed]
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812-21. [Crossref] [PubMed]
- Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016;149:631-8. [Crossref] [PubMed]
- Fermin AM, Afzal U, Culebras A. Sleep in Neuromuscular Diseases. Sleep Med Clin 2016;11:53-64. [Crossref] [PubMed]
- Smith PE, Edwards RH, Calverley PM. Ventilation and breathing pattern during sleep in Duchenne muscular dystrophy. Chest 1989;96:1346-51. [Crossref] [PubMed]


