High rate of arterial complications in patients supported with extracorporeal life support for drug intoxication-induced refractory cardiogenic shock or cardiac arrest
Introduction
Drug intoxication still represents a potentially lethal condition despite a better knowledge of the pathophysiologic mechanisms and a significant improvement of its management over the past 40 years (1). The clinical spectrum of poisoning from cardiotoxic drugs is broad encompassing life-threatening cardiac arrhythmias, hypotension or even cardiogenic shock and cardiac failure remains a leading cause of death (2).
Extracorporeal life support (ECLS) emerged in the last decade as an effective therapeutic option for cardiogenic shock patients refractory to optimal conventional treatment (3). So as stated in the toxicologic-oriented advanced cardiac life support guidelines, the use of mechanical circulatory support (MCS) should be an option in the management of drug-induced cardiogenic shock refractory to maximal therapy (4).
The analysis of the scientific literature shows a paucity of data about the use of ECLS in cardiotoxic drugs poisoning and, to the best of our knowledge, the majority of the published studies on this topic are case reports (2) or case series with a small number of patients (5-8). So, the aim of this report is to present the results of ECLS in the setting of drug intoxication in a single-center experience.
Methods
Study design
We performed an observational analysis of our prospectively collected database of ECLS utilization for drug intoxication at our institution. In accordance with national regulations on “non-interventional clinical research” (articles L.1121-1 and R.1121-2 of the French Public Health Code), authorization from an ethics committee and written informed consent from participants were not required.
Patient population
We included in this analysis consecutive patients who received an ECLS for refractory cardiogenic shock or in-hospital cardiac arrest due to poisoning from cardiotoxic drugs from January 2010 to December 2015. According to the European Society of Cardiology guidelines, cardiogenic shock was defined as hypotension (systolic blood pressure <90 mmHg) despite adequate filling status with signs of hypoperfusion (9). Refractory cardiac arrest was defined as the lack of return of spontaneous circulation within a period of at least 30 min of cardiopulmonary resuscitation under medical direction in the absence of pre-existing hypothermia (10). Age >65 years was a relative contraindication to ECLS implantation while pre-existing irreversible neurological damages or major comorbidities compromising short-term (<1 year) life expectancy were absolute contraindications.
ECLS technique and management
The technique of ECLS implantation and its management at our institution were previously described (11). Briefly, our ECLS team was composed by: (I) a senior cardiac surgeon and a resident in cardiac surgery for the implantation of the temporary MCS; (II) a senior anaesthesiologist of our Department of Anaesthesia and Intensive Care Unit for the perioperative medical management of the patient; (III) a technician for the preparation and priming of ECLS; and (IV) two nurses assisting the surgical team during ECLS implantation. The implantation of ECLS was performed in a surgical manner. The venous (Maquet, Rastatt, Germany; 25 and 29 French) and arterial (Maquet, Rastatt, Germany; 15, 17 and 19 French) cannulae were placed using a modified Seldinger technique after surgical exposure of the femoral vessels at the groin. As per institutional policy, an arterial catheter was systematically placed distally to the entry site of the arterial cannula to prevent lower limb ischemia. The ECLS system was also composed of venous and arterial heparinized polyvinyl chloride tubing, a membrane oxygenator (Quadrox Bioline, Jostra-Maquet, Orléans, France), a centrifugal pump (Rotaflow, Jostra-Maquet, Orléans, France) and an oxygen/air blender (Sechrist Industries, Anaheim, CA, USA). An intravenous bolus of 5,000 IU of unfractionated heparin was administered during ECLS implantation immediately before the cannulation of the femoral vessels. We did not use heparin in case of ECLS implantation during cardiac massage for refractory in-hospital cardiac arrest because of the presence of spontaneous coagulation abnormalities. At the admission to our intensive care unit target unfractionated heparin anti-Xa factor activity was maintained between 0.30 and 0.35 IU/mL during ECLS support.
ECLS flow was initially set at the theoretical cardiac output owing to the patient’s body surface area. However an inotropic support was used in order to maintain a left ventricular ejection with aortic valve opening, thus reducing the risk of pulmonary edema and intracardiac thrombosis. Moreover a vasopressor support was usually added to maintain a mean blood pressure of 65–70 mmHg.
The neurological evaluation was performed after a 24-h period of ECLS support. Serial transesophageal echocardiography controls were performed after progressive reduction of pump flow to assess the myocardial recovery. Patients stable during reduction trials and with left ventricular ejection fraction >25% and time-velocity integral >10 cm were weaned from ECLS (12). The decannulation procedure was performed in a surgical manner with reopening of the operative field at the groin. The venous cannula was removed over a purse-string suture of polypropylene 4/0 while the distal limb reperfusion catheter over a simple stich of polypropylene 5/0. Finally, after control of the femoral artery by passing proximal and distal shoestring ties around it, the arterial cannula was removed and the artery was reconstructed with interrupted sutures of polypropylene 6/0. Successful weaning was defined as ECLS decannulation without the need for reinsertion of ECLS or mortality within 48 h. If the weaning trial was not hemodynamically tolerated and the echocardiographic criteria were not fulfilled, patients with complete neurological recovery were directed to long-term ventricular assist device implantation or heart transplantation depending on age, general clinical and functional status and end-organ (respiratory, hepatic and renal) function. Conversely, ECLS support was considered futile and then stopped in the presence of multiple organ failure or brain death.
Outcome and statistical analysis
Pre-implantation, perioperative and post-implantation data were collected from the computerized medical charts of our hospital. The primary endpoint of our study was survival to hospital discharge with good neurological recovery after ECLS support. The neurological evaluation was performed using the Cerebral Performance Categories (CPC) score and good neurological recovery was defined as a CPC score of 1 or 2 on a 5-point scale (1= good cerebral performance, 2= moderate cerebral disability, 3= severe cerebral disability, 4= coma or vegetative state, 5= brain death or death). The secondary endpoints were complications rate during ECLS support, successful weaning rate from ECLS, intensive care unit and total hospital length of stay. Statistical analysis was performed using SPSS software, version 23.0 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation while categorical variables were presented as counts and percentages.
Results
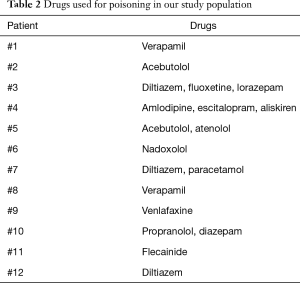
During the study period we performed 12 ECLS. The mean age of our patient population was 44.2±17.8 (range, 14–71) years with a predominance of females (66.7%). Table 1 shows the baseline characteristics of our study population. Nine (75%) patients presented a psychiatric disorder under medical treatment and 6 (50%) tried at least one previous suicide attempt. Calcium channel blockers and/or beta-blockers accounted for 10 (83.3%) drug intoxications while drugs with membrane stabilising activity were used in 8 (66.7%) cases. Table 2 presents the drugs used in our cases of poisoning. Every patient (100%) needed an inotropic and/or vasopressor support at the admission to our intensive care unit while 4 (33.3%) patients required the implantation of a percutaneous temporary pacemaker because of extreme bradycardia or complete atrioventricular block.

Full table
Full table
Table 3 summarizes the biological profile before ECLS implantation. The laboratory values were typical of cardiogenic shock state with tissue hypoperfusion as witnessed by the impairment of hepatic and renal function and the elevation of lactate levels. Mean left ventricular ejection fraction at the admission to our intensive care unit before ECLS implantation was 18.3%±7.6% (range, 10–25%).
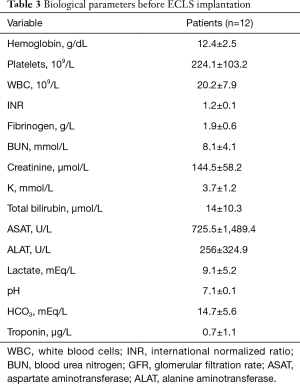
Full table
Nine (75%) patients developed cardiogenic shock not responsive to conventional treatment while ECLS was implanted during cardiopulmonary resuscitation for refractory in-hospital cardiac arrest in the remaining 3 (25%; mean no-flow time =0 min, mean low-flow time =67.5 min). The mean time from admission to ECLS implantation was 16±8.5 (range, 3–30) h.
Three (25%) patients (in-hospital cardiac arrest n=2, cardiogenic shock n=1) died while on ECLS during the first 48 hours of support [brain death n=2 (16.7%), multiple organ failure n=1 (8.3%)]. Nine (75%) patients were successfully weaned after a mean ECLS support of 2.4±1.1 (range, 1–5) days and then extubated after a mean mechanical ventilation of 6.7±2.4 (range, 3–10) days.
Four (33.3%) patients required continuous veno-venous hemofiltration during mechanical support and 2 (16.7%) of them continued it after ECLS weaning (maximum duration of hemofiltration =25 days). One (8.3%) patient experienced massive ischemic stroke with cerebral edema due to left middle cerebral artery infarction during the first 24 h of ECLS support and died the following day. Three (25%) patients suffered ventilator-associated pneumonia during ECLS support and intensive care unit stay. The pathogens were Pseudomonas aeruginosa (n=2) and Klebsiella pneumoniae (n=1). Finally, 6 (50%) patients developed lower limb ischemia (Figure 1A). Each patient was managed with ECLS decannulation: 2 (16.7%) patients underwent a concomitant iliofemoral thrombectomy, 3 (25%) of them needed further fasciotomy due to the development of compartment syndrome (Figure 1B) and the remaining patient (8.3%) required an amputation above the knee as a consequence of irreversible ischemia (Figure 1C). There was no bleedings requiring surgical re-exploration at the ECLS insertion site or groin wound infections.
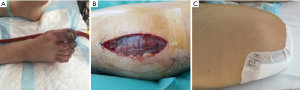
The length of stay in the intensive care unit was 7.4±7.2 (range, 1–26; median =5.5) days while 9 (75%) patients were discharged after a mean total hospital stay of 38.7±60.8 (range, 5–175; median =11) days. All the 9 survivors were discharged from hospital without neurological sequelae (CPC 1, n=9). The transthoracic echocardiography evaluation performed before hospital discharge showed a mean left ventricular ejection fraction of 56.6%±15.2% (range, 40–70%). The 30-day and 1-year survival for the patients who survived to hospital discharge was 75% and 75%, respectively.
Discussion
Intoxications with cardiovascular drugs represent a public health concern as they were involved in 4.6% of approximately 2.2 million poisonings in the USA in 2014 and accounted for 5.8% of the fatal cases (13). Moreover, severely poisoned patients could develop refractory cardiogenic shock or cardiac arrest that does not respond to maximal conventional treatment (14).
Refractory cardiogenic shock is now preferably supported with temporary MCS as a “bridge to decision” and this first-line strategy has been regularly highlighted in the last years by the Annual Reports of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) (15,16). Furthermore, in the specific setting of drug-induced cardiotoxicity, different experimental studies confirmed that ECLS offers better survival as compared to advanced cardiac life support in the management of acutely poisoned animals with membrane stabilising activity drugs (17,18).
ECLS is an effective rescue therapeutic option in refractory cardiogenic shock or cardiac arrest due to drug intoxication as previous articles reported an overall survival with good neurological recovery after ECLS support between 66% and 85% (2,5-8,19). Moreover, Masson et al. found a statistically significant survival benefit in ECLS-treated cardiotoxic drugs poisonings as compared to the conventional treatment (7). Mohan and coworkers reached the same conclusions in a comparative analysis of ECLS and standard supportive care in a population of drug intoxications caused by aluminium phosphide (8). Our results are consistent with these previous experiences as ECLS offered a survival to hospital discharge with good neurological recovery of 75%. Even the subgroup of critically ill patients with refractory in-hospital cardiac arrest (n=3) displayed a satisfactory survival rate (33%) after ECLS support. This is also comparable with the scientific literature as other investigators reported survival rates with good neurological outcome between 20% and 40% in the setting of ECLS for refractory in-hospital cardiac arrest of various etiologies (20-23).
However, temporary mechanical support with ECLS reveals some disadvantages. A recent meta-analysis of the literature with twenty studies and 1,866 patients identified a significant morbidity associated with ECLS including acute renal injury, bleeding, infections, lower limb ischemia and neurological complications (24). The rate of acute renal injury requiring continuous veno-venous hemofiltration (33%) and stroke (8%) experienced by our patients is consistent with this meta-analysis. Furthermore a retrospective analysis of the Extracorporeal Life Support Organization registry stated at approximately 4% the rate of ischemic stroke during ECLS support and a higher rate of cerebral infarction in extracorporeal cardiopulmonary resuscitation as compared with ECLS for cardiogenic shock (25). Finally, Schmidt and colleagues published a retrospective observational study on nosocomial infections in a large series of ECLS patients (n=220) (26). Ventilator-associated pneumonia was the most frequent (55%) ECLS-associated nosocomial infection and its incidence was quite greater than that observed in our small series.
Our study population presented a high rate (50%) of arterial complications. This incidence was obviously higher than that reported in the literature with a rate of arterial complications of 10–20% (27-32). The physiopathology of ipsilateral limb ischemia in patients supported with ECLS through a femoral cannulation is multifactorial and various predictors were identified over time such as younger age, pre-existing peripheral arterial disease and the absence of a distal perfusion catheter (27,28,30). Interestingly, gender, body surface area, body mass index, indication for temporary MCS (cardiogenic shock or cardiac arrest), implantation technique (surgical or percutaneous), cannula size and duration of ECLS support did not have a statistical correlation with the development of lower limb ischemia.
In our patients several factors could participate to the development of this disproportionate rate of arterial complications. Firstly, these patients were addressed to our Department of Cardiac Surgery in a critically ill situation with hemodynamic instability and peripheral hypoperfusion. The need for inotropic and vasoconstrictor support was exacerbated by the prevalent ingestion of beta-blockers or calcium channel blockers requiring a high vasoactive (noradrenaline) support. Finally, the presence of a nearly occlusive cannula in the common femoral artery and a potential, not known, underlying iliofemoral arteriopathy could also explain the high rate of lower extremity ischemia in our experience.
Arterial vascular complications are a relevant drawback of femoral ECLS but an integrated approach aiming at their prevention is still lacking.
The use of a distal antegrade reperfusion catheter in ECLS patients is still matter of debate. Some authors do not use it systematically but only expectantly, as they did not find a statistically significant difference with respect to mortality or limb ischemia in comparing a systematic and an expectant approach (27,31). Others implant the distal reperfusion only if the mean pressure in the superficial femoral artery 2 to 3 cm distal to the cannulation site is below 50 mmHg after ECLS initiation (33). We are of the opinion that the distal reperfusion catheter of the lower limb should be systematically put in the superficial femoral artery because it is technically simple and is performed only after cannulation of the femoral vessels and institution of the ECLS, thus not delaying the hemodynamic stabilisation of the patient. This attitude is shared by other teams involved in ECLS programs (28,30). There are other techniques for the prevention of lower limb ischemia which do not seem indicated in the routine daily practice [ligation of the posterior tibial artery and reperfusion of the distal extremity with a retrograde catheter (34), use of the dorsalis pedis artery for reperfusion of the leg (35)] or in urgent/emergent situations [arterial cannula inserted into a Dacron graft anastomosed to the common femoral artery (36)]. Finally the subclavian/axillary artery is an elegant solution in order to reduce the risk of lower limb ischemia (37). However, this approach presents some drawbacks. It is more time-consuming than the femoral approach and it does not seem suitable in a routine fashion even in the setting of cardiogenic shock. In one of the largest series published to date reporting the outcomes of axillary artery cannulation for ECLS the most frequent indication was after cardiotomy (61%), which could be considered a more stable cardiogenic shock state owing to the presence of the cardiopulmonary bypass (37). Moreover, it could cause a hyperperfusion syndrome of the upper extremity in approximately 25% of the patients (37).
In our series we managed lower limb ischemia with (I) ECLS decannulation in all cases owing to the high potential of myocardial recovery after drug intoxication and (II) the treatment of the underlying condition. In patients (n=2) with an embolic leg ischemia, we performed an iliofemoral thrombectomy while the remaining 4 patients with a low-flow, hypoperfusion state required either a fasciotomy (n=3) due to the presence of a compartment syndrome or an amputation (n=1). In those cases (not encountered in this case series) with ipsilateral lower limb ischemia in the absence of a complete myocardial recovery, our institutional policy is to switch the arterial cannulation site to the right subclavian artery via the interposition of a Dacron graft.
Limitations of the study
Our study displays of course several limitations. The small sample size is a limiting factor that could undermine the statistical power of our analysis. However our study population is comparable to that of previous articles of ECLS support for drug intoxication (5-8). Our conclusions are gathered from a single-center observational experience and may not be representative of the general population. We did not include in our analysis similar patients undergoing standard conventional treatment as a comparison group. Indeed, the objective of our work was not to evaluate the superiority of ECLS over the standard conventional treatment in the setting of drug intoxication-induced cardiogenic shock or cardiac arrest. Owing to the small sample size of each group, we did not perform a comparison between patients with and without lower limb ischemia in order to find a potential detrimental effect on survival. As per institution protocol, the absence of a percutaneous cannulation group did not allow for a comparison in term of vascular complications with the standard surgical cannulation technique.
Conclusions
Refractory cardiogenic shock or cardiac arrest due to cardiotoxic drug intoxication are life-threatening conditions which require a prompt intervention. ECLS represents an effective rescue therapeutic option in such a critical ill population as it offered to our patients an overall survival to hospital discharge with favourable neurological outcome of 75%. The overall complications rate was acceptable except for lower limb ischemia, as our study population exhibited a disproportionate rate (50%) of arterial complications. Further data are however necessary in order to best understand the possible relation between drug intoxication and lower limb ischemia, which was clearly superior to the reported rates for other ECLS indications.
Acknowledgements
None.
Footnote
Conflicts of Interest: Presented as a poster at the EuroELSO 2016, Glasgow (United Kingdom), June 1–4, 2016.
Ethical Statement: In accordance with national regulations on “non-interventional clinical research” (articles L.1121-1 and R.1121-2 of the French Public Health Code), authorization from an ethics committee and written informed consent from participants were not required.
References
- Baud FJ, Megarbane B, Deye N, et al. Clinical review: Aggressive management and extracorporeal support for drug-induced cardiotoxicity. Crit Care 2007;11:207-14. [Crossref] [PubMed]
- Johnson NJ, Gaieski DF, Allen SR, et al. A review of emergency cardiopulmonary bypass for fevere poisoning by cardiotoxic drugs. J Med Toxicol 2013;9:54-60. [Crossref] [PubMed]
- Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008;36:1404-11. [Crossref] [PubMed]
- Albertson TE, Dawson A, De Latorre F, et al. TOX-ACLS: toxicologic-oriented advanced cardiac life support. Ann Emerg Med 2001;37:S78-90. [Crossref] [PubMed]
- Babatasi G, Massetti M, Verrier V, et al. Severe intoxication with cardiotoxic drugs: value of emergency percutaneous cardiocirculatory assistance. Arch Mal Coeur Vaiss 2001;94:1386-92. [PubMed]
- Daubin C, Lehoux P, Ivascau C, et al. Extracorporeal life support in severe drug intoxication: a retrospective cohort study of seventeen cases. Crit Care 2009;13:R138. [Crossref] [PubMed]
- Masson R, Colas V, Parienti JJ, et al. A comparison of survival with and without extracorporeal life support treatment for severe poisoning due to drug intoxication. Resuscitation 2012;83:1413-7. [Crossref] [PubMed]
- Mohan B, Singh B, Gupta V, et al. Outcome of patients supported by extracorporeal membrane oxygenation for aluminum phosphide poisoning: An observational study. Indian Heart J 2016;68:295-301. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Conseil français de réanimation cardiopulmonaire, Société française d'anesthésie et de réanimation, Société française de cardiologie, et al. Guidelines for indications for the use of extracorporeal life support in refractory cardiac arrest. French Ministry of Health. Ann Fr Anesth Reanim 2009;28:182-90.
- Pozzi M, Koffel C, Armoiry X, et al. Extracorporeal life support for refractory out-of-hospital cardiac arrest: Should we still fight for? A single-centre, 5-year experience. Int J Cardiol 2016;204:70-6. [Crossref] [PubMed]
- Aissaoui N, Luyt CE, Leprince P, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 2011;37:1738-45. [Crossref] [PubMed]
- Mowry JB, Spyker DA, Brooks DE, et al. 2014 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 32nd Annual Report. Clin Toxicol (Phila) 2015;53:962-1147. [Crossref] [PubMed]
- de Lange DW, Sikma MA, Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clin Toxicol (Phila) 2013;51:385-93. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Transplant 2012;31:117-26. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Freedman MD, Gal J, Freed CR. Extracorporeal pump assistance - novel treatment for acute lidocaine poisoning. Eur J Clin Pharmacol 1982;22:129-35. [Crossref] [PubMed]
- Larkin GL, Graeber GM, Hollingsed MJ. Experimental amitriptyline poisoning: treatment of severe cardiovascular toxicity with cardiopulmonary bypass. Ann Emerg Med 1994;23:480-6. [Crossref] [PubMed]
- Massetti M, Bruno P, Babatasi G, et al. Cardiopulmonary bypass and severe drug intoxication. J Thorac Cardiovasc Surg 2000;120:424-5. [Crossref] [PubMed]
- Massetti M, Tasle M, Le Page O, et al. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg 2005;79:178-83. [Crossref] [PubMed]
- Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372:554-61. [Crossref] [PubMed]
- Kagawa E, Inoue I, Kawagoe T, et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation 2010;81:968-73. [Crossref] [PubMed]
- Wang CH, Chou NK, Becker LB, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest--a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation 2014;85:1219-24. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]
- Lorusso R, Barili F, Mauro MD, et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit Care Med 2016;44:e964-72. [Crossref] [PubMed]
- Schmidt M, Bréchot N, Hariri S, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis 2012;55:1633-41. [Crossref] [PubMed]
- Foley PJ, Morris RJ, Woo EY, et al. Limb ischemia during femoral cannulation for cardiopulmonary support. J Vasc Surg 2010;52:850-3. [Crossref] [PubMed]
- Bisdas T, Beutel G, Warnecke G, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg 2011;92:626-31. [Crossref] [PubMed]
- Aziz F, Brehm CE, El-Banyosy A, et al. Arterial complications in patients undergoing extracorporeal membrane oxygenation via femoral cannulation. Ann Vasc Surg 2014;28:178-83. [Crossref] [PubMed]
- Tanaka D, Hirose H, Cavarocchi N, et al. The Impact of Vascular Complications on Survival of Patients on Venoarterial Extracorporeal Membrane Oxygenation. Ann Thorac Surg 2016;101:1729-34. [Crossref] [PubMed]
- Ma RW, Huilgol RL, Granger E, et al. Does a distal perfusion cannula reduce ischaemic complications of extracorporeal membrane oxygenation? ANZ J Surg 2016;86:1002-6. [Crossref] [PubMed]
- Vallabhajosyula P, Kramer M, Lazar S, et al. Lower-extremity complications with femoral extracorporeal life support. J Thorac Cardiovasc Surg 2016;151:1738-44. [Crossref] [PubMed]
- Huang SC, Yu HY, Ko WJ, et al. Pressure criterion for placement of distal perfusion catheter to prevent limb ischemia during adult extracorporeal life support. J Thorac Cardiovasc Surg 2004;128:776-7. [Crossref] [PubMed]
- Hendrickson SC, Glower DD. A method for perfusion of the leg during cardiopulmonary bypass via femoral cannulation. Ann Thorac Surg 1998;65:1807-8. [Crossref] [PubMed]
- Kimura N, Kawahito K, Ito S, et al. Perfusion through the dorsalis pedis artery for acute limb ischemia secondary to an occlusive arterial cannula during percutaneous cardiopulmonary support. J Artif Organs 2005;8:206-9. [Crossref] [PubMed]
- Calderon D, El-Banayosy A, Koerner MM, et al. Modified T-Graft for Extracorporeal Membrane Oxygenation in a Patient with Small-Caliber Femoral Arteries. Tex Heart Inst J 2015;42:537-9. [Crossref] [PubMed]
- Chamogeorgakis T, Lima B, Shafii AE, et al. Outcomes of axillary artery side graft cannulation for extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2013;145:1088-92. [Crossref] [PubMed]


