Prognostic significance of sites of extrathoracic metastasis in patients with non-small cell lung cancer
Introduction
Metastatic disease across tumor histologies remains largely incurable despite significant recent advances in oncologic therapies (1). Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related deaths and represents a prime example of a malignancy for which metastatic disease has markedly worse outcomes as compared to non-metastatic disease (2). The concept of oligometastatic disease was introduced just over two decades ago and has been expanded to a multitude of cancer histologies including NSCLC (3,4). Notably, oligometastatic NSCLC may represent a disease state with limited disease burden amenable to localized therapy (i.e., resection, radiation, ablation) and improved survival outcomes (3-8) similar to that of locoregionally advanced NSCLC (9).
A recent single-institution retrospective review showed that patients with both synchronous and metachronous oligometastases from NSCLC had excellent overall survival (OS) (median OS =21.8 months) when the oligometastatic disease was treated radically either with surgery or stereotactic radiation (5). Improved OS was also seen in patients with NSCLC and synchronous limited brain metastases who had both their thoracic and intracranial disease treated (6). Lengthy OS has been shown in patients with oligometastatic lung cancer to a variety of organ sites (7).
However, to date, little comparative information exists to elucidate varying prognosis among patients with different sites of metastatic NSCLC. We aimed to use a large population-based database to investigate if, regardless of treatment paradigm, patients with specific sites of metastatic NSCLC had improved prognosis relative to other patients with M1b NSCLC. We hypothesized that metastatic disease to different organ sites would result in different survival outcomes, and surmised that tails on the survival curves would likely reflect survival of patients with unusually favorable histopathologic variants and/or oligometastatic disease.
Methods
The Surveillance, Epidemiology, and End Results-18 (SEER-18) database was queried for patients who were registered from 2010-2013 and diagnosed with metastatic NSCLC (10). SEER-18 incorporated TNM stage by American Joint Committee on Cancer (AJCC) 7th edition staging criteria in 2010; we chose not to include patients staged by earlier AJCC criteria given the significant changes in NSCLC staging, particularly for metastatic disease, introduced in 7th edition of the AJCC Staging Manual. NSCLC (and subcategory histologies) were defined via the following ICD-O-3 codes: adenocarcinoma (8140/3, 8255/3, 8260/3, 8310/3), bronchoalveolar carcinoma (8250/3–8254/3), adenosquamous carcinoma (8560/3), large cell carcinoma (8012/3), squamous cell carcinoma (8052/3, 8070-76/3, 8078/3, 8082-4/3), and NSCLC, not otherwise specified (NOS) (8046/3). All patients with M1b disease were included in this analysis; patients with unknown survival were excluded. We included patients with M1b disease as this represents patients with extrathoracic metastasis.
The extent and location of metastatic disease was determined through several fields in the Collaborative Stage Data Set used by the SEER-18 database. The “CS Mets at DX” code provides detailed information regarding location of metastatic disease (11). Specifically, code 40 indicates patients who have distant metastatic disease including those with metastasis to abdominal organs or other distant metastasis, and excluding those with distant lymph node disease, pericardial or pleural effusion, pleural disease, extension to the skeletal muscle, sternum, or chest skin, and intrapulmonary metastasis. It does include patients with carcinomatosis, distant metastasis not otherwise specific, and patients with a separate lesion in the chest wall or diaphragm. Additional codes for CS Mets at Dx-Brain, -Liver, and -Bone were used to further elucidate the location of distant metastatic disease. There is an additional code for CS Mets at Dx-Lung; however, given that we were assessing patients with primary lung malignancies and the inclusion of intrapulmonary metastasis within the AJCC 7th edition T and M staging for lung cancer, we chose to use this field only to verify that patients did not have intrapulmonary metastatic disease, thus all patients in this analysis had a value of 0 for this field. Any patient who did not have complete information for these four fields was excluded from further analysis. We separately grouped patients with brain metastases and without lung, liver, or bone metastases (brain-group); likewise we separately grouped those with liver and without lung, bone, or brain metastases (liver-group), and separately grouped those with bone and without lung, liver, or brain metastases (bone-group). Different combinations (i.e., brain-bone, brain-liver, liver-bone, and brain-liver-bone groups) were also categorized. Survival was compared to all other patients with M1b disease regardless of T or N stage.
Counts and percentages were used to describe categorical variables. Medians and interquartile ranges (IQRs) were used to describe continuous variables. Survival statistics were determined using Kaplan-Meier survival analyses. Both univariate and multivariate Cox proportional hazards models were used to determine the implications of various demographic and tumor characteristics on survival. All statistical analysis was performed using Stata 13 (College Station, TX, USA).
Results
Patient characteristics and impact on prognosis
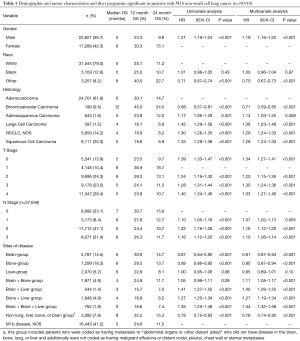
A total of 39,910 patients from the SEER-18 database met inclusion criteria. Details regarding patient demographics are shown in Table 1. Of note, the cohort was male (56.7%), Caucasian descent (79.0%), and had adenocarcinoma histology (61.9%). There was a spread of patients across various T and N stages (Table 1). The median follow-up period was 3 months (IQR 1–9 months). The median OS was 5 months (IQR 2–13 months).
Full table
Univariate and multivariate Cox proportional hazards analyses were performed using a variety of patient and tumor characteristics (Table 1). Male patients had a significantly worse prognosis than female patients on both univariate (HR =1.21, P<0.001) and multivariate (HR =1.19, P<0.001) analyses. Patients who had an ethnicity other than Caucasian or African-American had improved survival relative to those with Caucasian ethnicity on both univariate (HR =0.71, P<0.001) and multivariate (HR =0.70, P<0.001) analyses. As described in Table 1, patients with bronchoalveolar carcinoma had increased OS relative to those with adenocarcinoma; patients with all other histologies had decreased OS relative to those with adenocarcinoma on both univariate and multivariate analyses. Patients with a T stage other than T1 disease had decreased OS relative to those with T1 disease. Patients with any nodal disease had decreased OS relative to those with N0 disease.
Impact of sites of metastatic disease
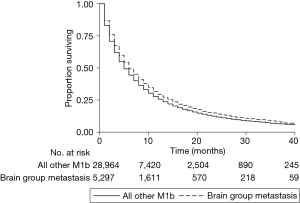
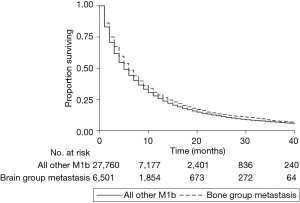
The distribution of sites of metastatic disease is shown in Table 1. A plurality of patients (41.2%) had M1b disease without documented evidence of distant metastasis to the brain, bone, liver, or lung. Of particular note to this analysis, 7.8% of patients had a CS Mets at DX code of 40 (see Methods for description) but did not have any documented brain, bone, liver, or lung metastases. This suggests that 7.8% of patients with a CS Mets at DX code of 40 have distant metastatic disease elsewhere in the body, and one could thus extrapolate that a similar percentage of patients in each anatomic grouping have an additional metastatic burden beyond the organs specified. Patients in the brain-group had improved survival relative to those with disease elsewhere on both univariate (HR =0.87, P<0.001, Figure 1) and multivariate (HR =0.91, P<0.001) analyses. Likewise, patients in the bone-group had improved survival relative to those with disease elsewhere on both univariate (HR =0.89, P<0.001, Figure 2) and multivariate (HR =0.90, P<0.001) analyses. Patients who had metastatic disease at organs not specified by the organ-specific codes also had improved OS relative to all others (Table 1). No other combination of organ metastases had improved OS relative to all other with M1b disease (Table 1).
Conclusions
We present this hypothesis-generating large population-based analysis of the prognostic significance of varying sites of synchronous oligometastatic disease in patients with NSCLC. This represents the largest study that we are able to identify to date that investigates this. We show that patients in the brain- and bone-groups represent populations that have improved OS relative to other patients with M1b disease. While the absolute increase in OS for both groups is only one month (median OS =6 months for brain-group and bone-group patients, median OS =5 months for patients with M1b disease, NOS), this represents a 20% increase in OS. Most significantly, this identifies a subset of patients with metastatic disease who have improved outcomes relative to other patients with metastatic NSCLC. Given the significant recent advances in the treatment of NSCLC using PD-1 inhibitors and the repeated studies showing their successes in treating metastatic NSCLC, especially to the brain, this offers further evidence that these patients represent a subset that may benefit from aggressive upfront therapy (12-16). We additionally show that patients who have diffusely metastatic disease to multiple organs have decreased OS relative to others with M1b disease, reinforcing the great strides still left to be made in the treatment of metastatic NSCLC. This is consistent with a prior report showing that patients with NSCLC metastatic to a single organ have improved OS relative to those with a more extensive disease burden (17). Further analysis of combining metastatic burden with genetic abnormalities present may further subcategorize patients with metastatic NSCLC (18).
We did not analyze the impact of various treatment modalities in this study given the limited information in the SEER database regarding treatment. Specifically, it would be impossible for us to ascertain using SEER information alone if a patient received radiation or surgery to the primary NSCLC, the site(s) of metastatic disease, or both. Further, we could not investigate the use of chemotherapy, as that is not recorded in the SEER database. The safety and efficacy of aggressive management of limited metastatic disease, especially among patients with metastases to brain and bone has been well-documented. Retrospective review of patients with a solitary synchronous brain metastasis showed that they could undergo cranial metastatectomy along with local treatment of their NSCLC without additional risk of complication or mortality. One-year OS in that cohort was 62% (19). Stereotactic radiosurgery (SRS) has also been shown to be safe and effective in control of intracranial disease when combined with local treatment of primary tumor (20-22). A small five-person prospective study in China of patients with synchronous solitary bone metastasis and primary NSCLC showed that simultaneous bone metastatectomy and lung cancer resection followed by chemotherapy was safe; no post-operative complications were seen. Median progression-free survival in this cohort was 13.2 months (23). Stereotactic body radiotherapy (SBRT) has been shown to be safe and efficacious in patients with oligometastatic lung cancer in a variety of additional anatomic locations (8,24-26). Further, a previous study of the SEER database showed that patients refusing palliative RT for metastatic NSCLC had diminished OS relative to those not refusing RT (27).
Our results also produced several other insights that are in agreement with previous literature. The majority of patients are male and the most frequent histology is adenocarcinoma (28). These results are also in concordance in broader studies of patients with any stage NSCLC in that male patients have decreased OS relative to female patients and Caucasian and African-American patients have decreased OS relative to those with other ethnicities (29). Of note, previous analysis of the SEER database has shown that among the “Other Ethnicity” category, there are disparities; patients with Asian heritage have improved survival relative to those with Native American heritage (30). We have reaffirmed that T stage remains a prognostic factor even in those with metastatic disease (31). Additionally, we have shown that patients with advanced nodal involvement have worse outcomes than those without nodal involvement, in concordance with previous reports in patients with metastatic NSCLC (18).
This analysis is limited by many factors that consistently limit others that use the SEER database. There is a limited amount of information that one can ascertain from the SEER database and there is likely some level of error in coding in individual patients; however the large number of patients that we have in the study will mitigate that somewhat. Specific to this study, we attempt to isolate patients with metastasis to specific organs. Presumably, most patients in a specific group (bone, brain, liver) had metastases confined to that organ, although patients may have harbored other sites of metastases not specifically recorded by the SEER program (most common site not recorded is likely adrenal). We do show that only 7.8% of patients who were coded as not having brain, bone, or liver metastasis were also coded as having distant metastatic disease in the absence of nodal disease, malignant effusions, or chest wall extension, suggesting that approximately 8% of patients in each grouping of organ-site metastasis had macroscopic disease elsewhere in the body.
Our methodology has been used previous to analyze the patterns of metastatic disease in patients with colorectal cancer (32). This study is also limited by a relatively small median follow-up time of only three months, though this is not surprising as we only assessed patients with M1b disease who have a very poor prognosis at diagnosis. We also could not assess the impact of metachronous metastasis as within the SEER database we can only investigate disease at time of diagnosis. Likewise, we could not analyze time to disease progression or the anatomic spread of disease progression as that information is not in the SEER database. Further, we could not assess the disease burden, either number of metastases or volume of disease, within each organ identified.
In this large population-based hypothesis-generating analysis of patients with M1b NSCLC using the SEER database, we have shown that patients with disease of the brain or bone alone in the absence of malignant effusions or liver, lung, chest wall, or distant nodal metastases have improved survival relative to those patients with metastatic disease elsewhere in the body. Given the incredible recent advances in both local and systemic treatment of metastatic disease, these patients may represent a population who could potentially benefit greatly from aggressive treatment of their upfront disease; further prospective study should investigate the ideal treatment options for these patients.
Acknowledgements
We thank Mrs. Laura Finger for editorial assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The SEER database is publicly accessible for those users who sign a SEER Research Data Agreement. No identifying information was collected regarding any patient; as such this study was exempt from Institutional Review Board approval.
References
- Turajlic S, Swanton C. Metastasis as an evolutionary process. Science 2016;352:169-75. [Crossref] [PubMed]
- de Groot PM, Carter BW, Betancourt Cuellar SL, et al. Staging of lung cancer. Clin Chest Med 2015;36:179-96. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [PubMed]
- Fleckenstein J, Petroff A, Schafers HJ, et al. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer 2016;16:348. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Patel AN, Simone CB 2nd, Jabbour SK. Risk factors and management of oligometastatic non-small cell lung cancer. Ther Adv Respir Dis 2016;10:338-48. [Crossref] [PubMed]
- Bergsma DP, Salama JK, Singh DP, et al. The evolving role of radiotherapy in treatment of oligometastatic NSCLC. Expert Rev Anticancer Ther 2015;15:1459-71. [Crossref] [PubMed]
- Cheruvu P, Metcalfe SK, Metcalfe J, et al. Comparison of outcomes in patients with stage III versus limited stage IV non-small cell lung cancer. Radiat Oncol 2011;6:80. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973-2012 varying). In: National Cancer Institute D, Surveillance Research Program, Surveillance Systems Branch (ed). (Released April 2015, based on the November 2014 submission). Available online: http://www.seer.cancer.gov
- Lung CS Mets at Diagnosis. Available online: http://web2.facs.org/cstage0205/lung/Lung_hab.html
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Hendriks LE, Derks JL, Postmus PE, et al. Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: results from a population-based study. Eur J Cancer 2015;51:2534-44. [Crossref] [PubMed]
- He YY, Zhang XC, Yang JJ, et al. Prognostic significance of genotype and number of metastatic sites in advanced non-small-cell lung cancer. Clin Lung Cancer 2014;15:441-7. [Crossref] [PubMed]
- Cheufou DH, Welter S, Chalvatzoulis E, et al. Surgery of primary lung cancer with oligometastatic m1b synchronous single brain metastasis: analysis of 37 cases. Thorac Cardiovasc Surg 2014;62:612-5. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Bougie E, Masson-Cote L, Mathieu D. Comparison between surgical resection and stereotactic radiosurgery in patients with a single brain metastasis from non-small cell lung cancer. World Neurosurg 2015;83:900-6. [Crossref] [PubMed]
- Parlak C, Mertsoylu H, Guler OC, et al. Definitive chemoradiation therapy following surgical resection or radiosurgery plus whole-brain radiation therapy in non-small cell lung cancer patients with synchronous solitary brain metastasis: a curative approach. Int J Radiat Oncol Biol Phys 2014;88:885-91. [Crossref] [PubMed]
- Zhao T, Gao Z, Wu W, et al. Effect of synchronous solitary bone metastasectomy and lung cancer resection on non-small cell lung cancer patients. Oncol Lett 2016;11:2266-70. [PubMed]
- Agolli L, Valeriani M, Nicosia L, et al. Stereotactic ablative body radiotherapy (SABR) in pulmonary oligometastatic/oligorecurrent non-small cell lung cancer patients: a new therapeutic approach. Anticancer Res 2015;35:6239-45. [PubMed]
- Chawla S, Chen Y, Katz AW, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys 2009;75:71-5. [Crossref] [PubMed]
- Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008;112:650-8. [Crossref] [PubMed]
- Stavas MJ, Arneson KO, Ning MS, et al. The refusal of palliative radiation in metastatic non-small cell lung cancer and its prognostic implications. J Pain Symptom Manage 2015;49:1081-7.e4. [Crossref] [PubMed]
- Moumtzi D, Lampaki S, Zarogoulidis P, et al. Prognostic factors for long term survival in patients with advanced non-small cell lung cancer. Ann Transl Med 2016;4:161. [Crossref] [PubMed]
- Morgensztern D, Waqar S, Subramanian J, et al. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol 2009;4:1524-9. [Crossref] [PubMed]
- Berz D, Faricy-Anderson KE, Weitzen S, et al. Do race and ethnicity predict survival in metastatic non-small cell lung cancer? Med Health R I 2010;93:299-302. [PubMed]
- Collaud S, Stahel R, Inci I, et al. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer 2012;78:234-8. [Crossref] [PubMed]
- Qiu M, Hu J, Yang D, et al. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget 2015;6:38658-66. [Crossref] [PubMed]


