Two cases of the bronchial carcinoid tumors successfully treated with the parenchymal-sparing bronchoplastic resections
Introduction
Neuroendocrine tumours (NETs) typically arise in the gastrointestinal tract, pancreas, lung and thymus. Pulmonary NETs are a distinct subset of tumors accounting for approximately 20% of all primary lung cancers (1). The 2015 World Health Organization (WHO) Lung Tumors Classification combined architectural patterns (cell size, organoid palisading rosettes, distinct nuclear/cytoplasm ratio) with other parameters (different mitotic index, presence of necrosis), aiming at classifying pure pulmonary NETs in four different types: typical carcinoid (TC), atypical carcinoid (AC), large-cell neuroendocrine carcinoma (LCNC) and small-cell lung carcinoma (SCLC) (2). Carcinoid tumors account for 2% of primary lung tumors (2,3). Definitely more frequent occurs TC tumors (2,3). There are found mostly in the 5th and 6th decade of life, more often in women (4). AC tumors are more common in the older age group than TC (average age about 57 years), more often in men (3,4). Carcinoid tumors differ in location, size, presence of necrosis and mitotic activity (3,5,6). About 30% of patients with lung carcinoid initially do not show any clinical signs (7). Symptoms occur sooner in patients with the central tumor bronchial location, e.g. cough (32%), hemoptysis (26%), pneumonia (24%) (3,8-10). Carcinoid syndrome is found in about 2–3% of patients (3,11). Cushing’s syndrome, acromegaly, hypercalcaemia or hypoglycemia affect a small group of patients (9-11). In the diagnosis of carcinoid IHC is very important (12). The most effective, radical treatment carcinoid tumors is surgery (11,13). We present the cases of two patients with bronchial carcinoid efficiently treated with the parenchymal-sparing bronchoplastic procedures.
Case presentation
Case 1
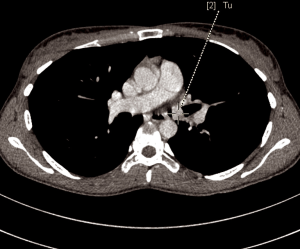
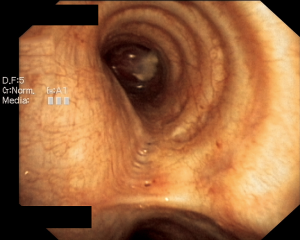
A 28-year-old woman was admitted to the Department of the Internal Medicine of the 4th Military Hospital in Wroclaw, Poland, because of dyspnoea. Since two years she has been under the Pulmonary Clinic care with suspected bronchial asthma. There was no significant clinical improvement with applied treatment (combination of inhaled corticosteroids and long-acting beta-2 agonists). Because of worsening shortness of breath, including rest, the patient was referred to the hospital diagnosis. At the time of the current admission to the hospital the physical examination revealed: tachycardia 110/min and wheezing over the lungs. The patient underwent diagnostics. The routine blood tests showed no abnormalities. The arterial blood gas showed hypoxaemia. The respiratory functional study revealed the forced expiratory volume in one second (FEV1) of 2.03 L (actual); 3.46 L (predicted normal); 58.75% (% of predicted). The moderate-to-severe degree of the obstruction with lung hyperinflation, the increased airway resistance and mild decrease in DLCO (the carbon monoxide diffusion capacity) were diagnosed. The CT scans of the chest have shown the left main bronchus tumor size 1.5 cm (AP) × 1.3 cm (TR) × 1.5 cm (CC) with one bronchopulmonary lymph node (Figure 1). The bronchoscopy has confirmed in the left main bronchus, below the carina of about 2.5–3 cm, smoothly contoured tumor with a vessels net, surrounded by necrotic and purulent masses (Figure 2). Additionally, the patient underwent octreotide scan, gastroscopy, colonoscopy and the abdominal and pelvis MRI to exclude any metastases. The levels of laboratory studies such as chromogranin A, 24-hour 5 hydroxyindoleacetic acid (5-HIAA) and tumor markers were not elevated.
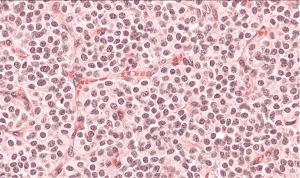
The patient was qualified for surgical treatment. The sleeve bronchiectomy, i.e., the partial resection of the distal left main bronchus, with end-to-end anastomosis was performed. The intraoperative histopathological examination of the bronchial ring showed no neoplastic tissue or in the proximal or the distal margin. The final histopathology confirmed AC tumor with negative lymph nodes, G2, pT1aN0M0R0L0V0. The lymph nodes 7 (0/1), 11 (0/2) and 5 (0/2) were without tumor metastases. The IHC: NCAM (+), synaptophysin (+), chromogranin (+), index Ki-67 7%, 4/10 HPFs (Figure 3). The follow-up (chest CT and bronchoscopy) in the year after surgery revealed no local recurrence. The patient remains in the further observation for at least five consecutive years.

Case 2
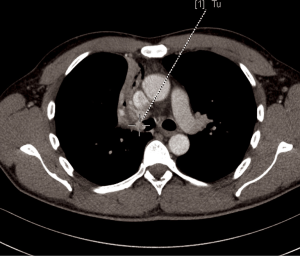
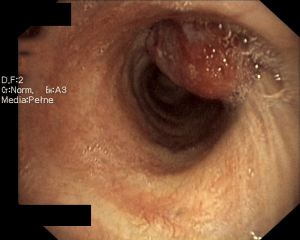
A 44-year-old man was admitted to the Department of the Internal Medicine of the 4th Military Hospital in Wroclaw, Poland because of cough, fever and hemoptysis. At the time of the current admission to the hospital the physical examination revealed only wheezing over the lungs. The patient underwent diagnostics. The routine blood tests showed increased markers of inflammation. The arterial blood gas showed hypoxaemia. The respiratory functional study revealed FEV1 (the forced expiratory volume in one second) of 3.00 L (actual); 3.48 L (predicted normal); 86.11% (% of predicted). The ventilatory impairments of the moderate obstruction type and the mild decrease in DLCO (the carbon monoxide diffusion capacity) were diagnosed. The total lungs capacity (TLC) and vital capacity (VC) were within normal limits. The CT scans of the chest have shown the right main bronchus tumor of size 1.0 cm × 1.1 cm with nodal reaction (Figure 4). The bronchoscopy has confirmed in the smoothly contoured tumor with a vessels net in the right main bronchus (Figure 5). The patient was qualified for sleeve right upper lobectomy with lymphadenectomy. The histopathological examination of intraoperative showed no neoplastic tissue or in the proximal or the distal margin. The final histopathology confirmed TC tumor with negative lymph nodes, G1, pT1N0M0R0L0V0, 1/10 HPFs (Figure 6). The lymph nodes 4R (0/4), 7 (0/3) and 10R (0/1) were without tumor metastases. The patient remains in the further observation for at least five consecutive years.
Discussion
In 1947 Price-Thomas performed the first sleeve resection of the bronchus to remove an adenoma originating in the right main bronchus. In 1952 Allison performed the first sleeve resection for lung cancer (14).
Bronchial carcinoids occur rarely. They comprise fewer than 3% of all lung tumors and are of low-grade malignancy (15). Chabowski et al. reported only 19 (5%) cases of carcinoid in two years period [1998–1999] with an excellent five-year survival rate of 94.7% (16). Kasprzyk et al. reported only three cases of carcinoid in five-year period [2001–2005] treated with sleeve procedures (14). Therefore, any prospective trials on bronchial carcinoids are difficult to perform.
The carcinoids are most often located centrally within the tracheobronchial tree. So, the presenting symptoms are usually related to mechanical obstruction of the bronchus by central tumors. The presence of the symptoms allows for early detection of the tumor. In our cases the symptoms were as follows: dyspnea, cough, hemoptysis and fever.
The lung saving resection, i.e., the avoiding pneumonectomy, remains the main treatment option for carcinoids as they are resistant to chemotherapy or radiotherapy on principle (17). The complete resection with disease-free margins is mandatory. Terzi et al. claimed that the bronchoplastic procedures are successful if a frozen section of the bronchial margins is negative, which is very important to reduce the risk of local recurrence and the risk of anastomotic dehiscence (17). Sternau et al. pointed out that the bronchoplastic resections are technically more difficult and more precise to perform in comparison with traditional lobectomy (18). TCs as less aggressive can be completely resected, sparing as much unaffected lung parenchyma as possible. ACs should be treated by radical resection, as other non-small cell lung cancer, because of possible lymph node involvement (15). Approximately 20% of carcinoids present as endobronchial polyp-like lesion without involvement of lung parenchyma (19). Main bronchial resection is regarded as an appropriate technique for selected malignant lesions that are limited to the main bronchus without lymphatic involvement. In the presented cases the second patient had TC without parenchyma involvement, so only the tumor with the ring of left main bronchus was resected. The first patient had AC with parenchyma infiltration, so the sleeve right upper lobectomy with lymphadenectomy was performed. In both cases the frozen sections of the bronchial margins were negative.
The postoperative follow-up visits are very important in order to diagnose potential stenosis in the anastomosis site.
Conclusions
Conservative lung resection with bronchoplasty for bronchial carcinoid seems to be a safe procedure with low morbidity and good long-term survival. The surgeon’s experience and appropriate bronchoplastic technique decrease the number of postoperative complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Chen LC, Travis WD, Krug LM. Pulmonary neuroendocrine tumors: what (little) do we know? J Natl Compr Canc Netw 2006;4:623-30. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- McMullan DM, Wood DE. Pulmonary carcinoid tumors. Semin Thorac Cardiovasc Surg 2003;15:289-300. [Crossref] [PubMed]
- Corrin B, Nicholson AG. Pathology of the lungs. Churchill Livingstone 2006:1-35:527-608.
- Franks TJ, Galvin JR. Lung tumors with neuroendocrine morphology. Essential radiologic and pathologic features. Arch Pathol Lab Med 2008;132:1055-61. [PubMed]
- Gosney JR. Neuroendocrine tumours of the lung. Current Diag Pathol 2000;6:64-70. [Crossref]
- Brambilla E, Lantuejoul S. Neuroendocrine neoplasms. In: Zander DS, Farver CF. editors. Pulmonary pathology. A volume in the series foundations in diagnostic pathology. Churchill Livingstone, 2008:563-77.
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [Crossref] [PubMed]
- Lim E, Goldstraw P, Nicholson AG, et al. Proceedings of the IASLC International Workshop on Advances in Pulmonary Neuroendocrine Tumors 2007. J Thorac Oncol 2008;3:1194-201. [Crossref] [PubMed]
- Warren WH, Welker M, Gattuso P. Well-differentiated neuroendocrine carcinomas: the spectrum of histologic subtypes and various clinical behaviors. Semin Thorac Cardiovasc Surg 2006;18:199-205. [Crossref] [PubMed]
- Morandi U, Casali C, Rossi G. Bronchial typical carcinoid tumors. Semin Thorac Cardiovasc Surg 2006;18:191-8. [Crossref] [PubMed]
- Lyda MH, Weiss LM. Immunoreactivity for epithelial and neuroendocrine antibodies are useful in the differential diagnosis of lung carcinomas. Hum Pathol 2000;31:980-7. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Nakatani Y, et al. Pulmonary large cell neuroendocrine carcinoma: its place in the spectrum of pulmonary carcinoma. Ann Thorac Surg 2007;84:702-7. [Crossref] [PubMed]
- Kasprzyk M, Dyszkiewicz W, Piwkowski C, et al. Early and late results after sleeve lobectomies in patients with non-small cell lung cancer. Kardiochir Torakochir Pol 2009;6:366-71.
- Uchino K, Okada M, Sakamoto T, et al. Bronchoplasty for bronchial carcinoid tumor without removing lung parenchyma. Jpn J Thorac Cardiovasc Surg 2006;54:345-7. [Crossref] [PubMed]
- Chabowski M, Orłowski TM, Rabczenko D. Prognostic factors and efficacy of surgical treatment for non-small cell lung cancer analysis: department of surgery NTLDRI (1998–1999). Pneumonol Alergol Pol 2008;76:1-10. [PubMed]
- Terzi A, Lonardoni A, Feil B, et al. Bronchoplastic procedures for central carcinoid tumors: clinical experience. Eur J Cardiothorac Surg 2004;26:1196-9. [Crossref] [PubMed]
- Sternau A, Chwirot P, Tomaszewski D, et al. Bronchoplastic procedures for bronchial carcinoid tumors. Ann Acad Med Gedan 2006;36:175-81.
- Anile M, Daniele Diso D, Rendina EA, et al. Bronchoplastic Procedures for Carcinoid Tumors. Thoracic Surgery Clinics 2014;24:299-303. [Crossref] [PubMed]


