Respiratory support with venovenous extracorporeal membrane oxygenation during stent placement for the palliation of critical airway obstruction: case series analysis
Introduction
Fluoroscopic placement of self-expandable metallic stents (SEMSs) is generally considered to be safe, easy, and effective for palliation of malignant and benign airway obstructions (1-3). In cases of non-life threatening airway obstructions, topical anesthesia of the pharynx and larynx, procedural sedation, or general anesthesia are routinely performed for stent placement (1-5). However, in patients with critical airway obstruction (nearly complete obstruction due to severe stricture and/or sputum), airway stent placement can be extremely dangerous because the airway collapse limits the ability to oxygenate and ventilate during interventional procedures despite ventilation support (6-8).
Venovenous extracorporeal membrane oxygenation (VV ECMO) is used not only to support ventilation of patients suffering from respiratory failure (8-13), but also to manage hypoxic patients with critical airway obstruction during various procedures, such as stent placement and removal, rigid bronchoscopy, or pediatric surgery (6-9,14-16). However, previous reports focusing on stent placement under respiratory support with VV ECMO have been limited to case reports or very small series (6,7,14,15). Further larger scale studies on stent placement under respiratory support using VV ECMO in patients with critical airway obstruction are required to establish the indications for VV ECMO before the procedures and to elucidate its technical and clinical outcomes. The purpose of our current study was therefore to retrospectively evaluate the technical feasibility and safety of fluoroscopic stent placement under respiratory support with VV ECMO in patients with critical airway obstruction.
Methods
Patient population
The institutional review board approved the study and waived the requirement to obtain informed consent. Between December 2009 and December 2015, we retrospectively reviewed the records of 17 patients (14 male and 3 female patients; mean age: 63 years; range, 30–82 years) who underwent SEMSs under respiratory support using VV ECMO for critical airway obstructions caused by malignant (n=16) or benign (n=1) etiology.
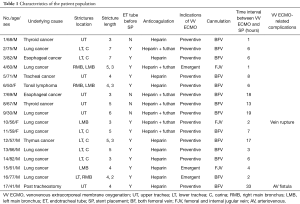
The diagnosis was established by bronchoscopic biopsy and/or chest computed tomography (CT). Airway obstruction was located in the upper trachea (n=6), in the lower trachea extending into the carina level (n=5), in multiple sites (n=4), or in the left main bronchus (n=2). An endotracheal tube was inserted in 13 of 17 patients before the procedure. In the remaining four patients, the endotracheal tube could not be inserted because the stricture was located in the upper trachea. The characteristics of the patient population in this study are summarized in Table 1.
Full table
VV ECMO
The decision to use of VV ECMO was made after full consultation with our multidisciplinary team, consisting of oncologists, interventional radiologists, and pulmonologists, that provides respiratory support during stent placement procedures.
In all cases, VV ECMO was established by the cardiovascular surgeons before stent placement. Two types of centrifugal pumps were used by the ECMO systems employed in this study: Capiox Emergency Bypass System (Terumo, Inc., Tokyo, Japan) and Centrifugal Rotaflow pump (Maquet Inc., Rastatt, Germany). Vascular cannulation was established with two cannulation approaches including outflow cannula via right internal jugular vein and inflow cannula via femoral vein or both femoral veins for outflow and inflow cannulas using 17-Fr to 28-Fr venous cannulae (RMI, Edwards Lifesciences LLC, Irvine, CA, USA). Before application of ECMO, all patients received intravenous injection of heparin. In some patients, continuous infusion of futhan was performed to maintain the activated clotting time at 130 to 150 seconds. The patients were weaned off the VV ECMO support gradually when the oxygen fraction of the oxygenator was less than 30% and after flexible bronchoscopy revealed airway patency.
Stent placement and removal
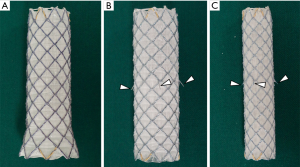
The stents used in this study have been described in detail in earlier studies (1-3). Two types of SEMS (S&G Biotech, Seongnam, Korea) were used for the trachea in this study (Figure 1): a straight barbed SEMS for the upper and middle trachea and bronchus and a flared end SEMS without barbs for the lower trachea.
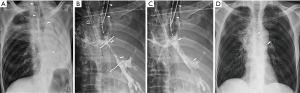
The site, severity, and length of the stricture were evaluated before stent placement using chest CT and bronchoscopy. Under respiratory support with VV ECMO, a 180-cm, 0.035-inch exchange guide wire (Radifocus M; Terumo, Tokyo, Japan) was inserted through an endotracheal tube across the stricture into the distal trachea or bronchus under fluoroscopic guidance. If there was no endotracheal tube, primary assessment of the airway was performed by a pulmonologist using a bronchoscope that was kept on standby in the angiography suite. After identifying the lesion, the guide wire was passed through the bronchoscope and positioned distal to the lesion under fluoroscopic guidance. The bronchoscope was then removed, leaving the guide wire in place. A straight, 5F, graduated-sized catheter (Cook, Bloomington, IN, USA) was passed over the guide wire to the distal portion of the stricture in order to measure its length. If necessary, the location of the narrowed tracheobronchial lumen was marked on the patient’s skin using fluoroscopic guidance. When a stricture was not well-defined by fluoroscopy, the guide wire was removed from the catheter and a small amount of contrast medium (Omnipaque 300, GE healthcare, Cork, Ireland) was injected through the catheter in order to opacity the narrowed lumen, after which the length of the stricture was measured. Using fluoroscopic guidance, the entire introducer set was passed over the guide wire and advanced until the distal tip of the stent reached beyond the stricture. A stent that was longer than the stricture of at least 1–2 cm each end was then placed (Figure 2).
To make the stent removable, a nylon loop 2 mm in diameter was hooked inside each bend of the upper end of the stent, and two nylon threads were passed through each of these nylon loops to form a larger loop (drawstring) that filled the inside circumference of the proximal end of the stent. The stent retrieval set consisted of a 13-French sheath, a 10-French dilator, and a hook wire. The end of the retrieval hook wire was shaped like a question mark to hook the stent drawstring, as previously described (1-3).
Definition and analysis of data
We evaluated technical and clinical success, indications for VV ECMO before stent placement, Hugh-Jones dyspnea grade before and after stent placement, duration of hospital stay, and complications related to the procedure. Technical success was defined as successful stent placement in the proper position. Clinical success was defined as an increase of at least one grade in the Hugh-Hones dyspnea grade and ECMO weaning, and for patients with an endotracheal tube, successful extubation within 7 days after stent placement. Failure of mechanical ventilation was defined as a failure to provide sufficient oxygenation despite optimal mechanical ventilation. Orthopnea was defined as development of respiratory distress in the supine position.
The Hugh-Jones dyspnea grade was used to evaluate the severity of dyspnea before and 7 days after stent placement in all patients. The Hugh-Jones dyspnea grade (17) was defined as follows: (I) patient’s breathing is as good as other people of the same age and build during work, walking, and climbing hills or stairs; (II) patient is able to walk at the same pace as normal people of same age and build on level ground, but is unable to keep up on hills or stairs; (III) patient is unable to keep up with normal people on level ground, but is able to walk about a mile or more at his/her own speed; (IV) patient is unable to walk more than about 50 yards on level ground without a rest; (V) patient is breathless on talking or undressing, or unable to leave the house because of breathlessness. The Hugh-Jones Grade before and 7 days after stent placement were analyzed with the Wilcoxon signed rank test and overall survival was calculated according to the Kaplan-Meier method. Statistical analysis was performed using SPSS (version 23; SPSS, Chicago, IL, USA). A two-sided P value of less than 0.05 was considered to indicate statistical significance.
Results
VV ECMO before stent placement
Indications for VV ECMO support before stent placement included failure of mechanical ventilation in 13 (76.5%) patients, and orthopnea in 4 (23.5%) patients. Three of 13 patients with failure of mechanical ventilation underwent emergency VV ECMO, because of a sudden worsening of their dyspnea. In four patients with orthopnea, an endotracheal tube could not be placed because the stricture was located near the vocal cord. These patients could not lie in the supine position because of their marginal oxygen saturation and persistent acidosis. Anticoagulation management was performed only intravenous infusion of heparin (3,000–5,000 IU) in 10 patients, and additionally, continues infusion of futhan in seven patients. The cannulas were placed into the both femoral vein in 14 patients or into the femoral vein for infusion and the right internal jugular vein for drainage in the remaining three patients. All patients were referred for stent placement to the department of interventional radiology 1–33 hours (mean: 9 hours) after VV ECMO implementation.
During the mean ECMO time of 42 hours (range, 5–121 hours), ECMO-related complications developed in two (11.7%) patients. In one patient (No. 10), a right femoral vein rupture and hematoma occurred during insertion of the ECMO tube and a stent graft was placed to prevent further extravasation. In another patient (No. 17), a fistula between the superior femoral artery and the adjacent femoral vein was detected by angiography 3 days after ECMO implementation. A stent graft was then successfully placed to seal off the fistula in this case.
Technical and clinical outcomes
Fluoroscopic placement of a SEMS was technically successful in all 17 study patients (100%) with no procedure-related complications. 15 (88.2%) of these 17 patients showed one or more grades of improvement on the Hugh-Jones grade and underwent ECMO weaning, including 11 patients with successful extubation. The Hugh-Jones grade significantly improved 7 days after stent placement (from 4.7±0.4 to 3.1±0.9, P<0.001). Extubation was possible in 11 (84.6%) of 13 patients at 0–6 days (mean: 3 days) after stent placement. VV ECMO weaning was successful in all patients 2–87 hours (mean: 33 hours) after stent placement. However, two cases (No. 12 and 13, 11.8%) showed no improvement in dyspnea, necessitating the maintenance of the endotracheal tube until their death (24 and 25 days, respectively).
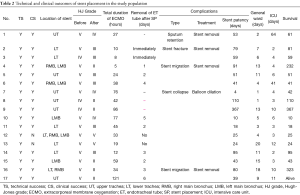
A total of five (29.4%) complications occurred at 4–91 days (mean: 61 days) after stent placement. These complications included stent migration in two patients (11.7%), sputum retention in one (5.9%), stent fracture in one (5.9%), and stent collapse in one (5.9%). The stents were removed in four (80%) of five patients at 53–91 days (mean: 76 days) after stent placement. Respiratory support using VV ECMO was not necessary during the stent removal. Two (No. 4 and 16) of those four patients did not require additional treatment for improvement of their stricture until their death. The remaining two patients underwent a tracheostomy immediately after stent removal because of recurrence of dyspnea. These patients died 2 (No. 2) and 12 (No. 1) days after the tracheostomy, respectively. In the patient (No. 7) with suffered a stent collapse, balloon dilation was performed to expand the stenotic portion of the stent; however, this patient died 42 days after stent placement due to near total obstruction of the trachea caused by recurring stent collapse accompanied by wire fracture. The mean of hospital stay duration was 19 days (range, 4–66 days) including 8 days (mean, range, 1–20 days) in general ward and 11 days (mean, range, 2–64 days) in intensive care unit (ICU). The technical and clinical outcomes and the complications after stent placement in our current patient series are summarized in Table 2.
Full table
Survival periods
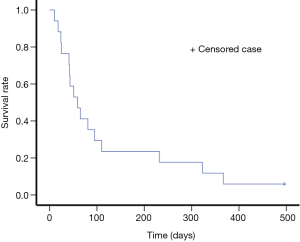
During the mean follow-up period of 123 days after stent placement (range, 10–496 days), 16 of the 17 patients died because of progress underlying disease. The median and mean survival periods were 59 days [95% confidence interval (CI), 29–89 days] and 122 days (95% CI, 56–189 days) (Figure 3).
Discussion
Respiratory support to optimize oxygenation is an important issue during interventional procedures for critical airway obstruction. Various ventilation modes, including maintenance of spontaneous ventilation, intermittent positive pressure ventilation via a side port of a rigid bronchoscope, and low- or high-frequency jet ventilation have been introduced (18,19). Both spontaneous ventilation and intermittent positive pressure ventilation via the ventilating port were not feasible in our present study series because of severe and complicated airway obstruction. Thus, we established VV ECMO before stent placement in these patients. VV ECMO provides time to plan and implement adequate treatment, thereby minimizing procedure-related complications. Furthermore, interventional radiologists feel more comfortable using VV ECMO when performing a high-risk procedure.
Our present study findings showed that stent placement under respiratory support using VV ECMO was successfully performed in all patients. Furthermore, extubation was possible in 11 (84.6%) of 13 patients with an endotracheal tube and ECMO was successfully removed from all patients after stent placement. Only two of our patients (15.4%) required maintenance of the endotracheal tube until their death. The complication rates of stent placement and VV ECMO were 29.4% and 11.7%, respectively, but all of these complications were successfully managed by additional interventional procedures without surgery. Considering the high morbidity and mortality associated with surgeries such as tracheal resection or reconstruction in patients with critical airway obstruction, SEMS placement under respiratory support with VV ECMO may be the best therapeutic alternative to prolong survival with relief of dyspnea in such cases.
ECMO is typically used as a rescue strategy for patients with respiratory and/or cardiovascular failure. ECMO sustains life following acute lung failure, allowing enough time for diagnosis, treatment, and recovery. Peek et al. (12) reported previously that the use of ECMO for severe adult respiratory failure produced significant increase in lung-protective ventilation and overall survival compared with conventional ventilation. In addition, ECMO can provide sufficient oxygenation during airway interventional procedures, regardless of the patient position. However, ECMO-related complication rates of 24–55% have been reported, including potentially life-threatening complications such as bleeding, hemolysis, air leakage, and thrombosis (20,21). In our current study, cannulation-related complications of VV ECMO occurred in two (11.7%) patients, and those were successfully managed with a fully covered stent graft without surgery. In these patients, placement of both airway stent and stent graft was performed at the same time by interventional radiologists to manage both ECMO-related complications and critical airway obstruction. Thus, although conventional ventilation is sufficient for a majority of patients undergoing airway intervention, ECMO can be used in a safe and effective manner during this procedure in a subset of patients with critical airway obstruction.
Rigid bronchoscopy can be an effective tool to alleviate airways obstruction in cases of malignant and benign diseases (22-24). During rigid bronchoscopy under procedural sedation or general anesthesia, intermittent positive pressure ventilation via a side port of a rigid bronchoscope is commonly performed to maintain oxygenation (19). However, during stent placement under fluoroscopic guidance, it is difficult to support oxygenation, and the patient’s position is very important for ensuring successful stent placement. VV ECMO was found in our current analysis to be useful as a bridge that supports high-risk stent placement as a lifesaving procedure. Conventional ventilation is sufficient for the vast majority of patients undergoing airway intervention; however, a subset of patients cannot maintain respiratory function during intervention and can be safely bridged with ECMO support.
In our study, mean survival period was 122 days (range, 10–496 days) after stent placement. In the 4 (23.5%) of 17 patients, they died within 30 days (mean, 19 days; range, 10–25 days) after stent placement because of a respiratory problem that associated with the underlying disease despite the successful ECMO weaning. It may promote criticism for cost-effectiveness and overtreatment for patients with poor clinical outcomes who underwent stent placement under VV ECMO. In addition, it may potentially prolong patients’ suffering. Further studies will be evaluated to include patients with standardized treatment and indications.
To our knowledge, we have here analyzed the largest series to date of SEMS placement under respiratory support via VV ECMO. The major limitation of our study was its single-center, retrospective design, and uncontrolled method. However, prospective, randomized, controlled trials may be difficult because these cases are rare and the procedure is usually performed in emergency situations. The small number of patients and short follow-up period represent other limitations of our current study.
In conclusion, fluoroscopic stent placement under respiratory support using a VV ECMO can be successfully performed in patients with critical airway obstruction, especially in cases of respiratory distress despite ventilation support and an inability to lie in the supine position. However, further studies will be needed to validate the standardized methods and specific indications.
Acknowledgments
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2014R1A2A2A01005857).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board of Asan Medical Center (No. S2017-0987-0001) and waived the requirement to obtain informed consent.
References
- Song HY, Shim TS, Kang SG, et al. Tracheobronchial strictures: treatment with a polyurethane-covered retrievable expandable nitinol stent-initial experience. Radiology 1999;213:905-12. [Crossref] [PubMed]
- Shin JH, Kim SW, Shim TS, et al. Malignant tracheobronchial strictures: palliation with covered retrievable expandable nitinol stent. J Vasc Interv Radiol 2003;14:1525-34. [Crossref] [PubMed]
- Kim JH, Shin JH, Song HY, et al. Benign tracheobronchial strictures: longterm results and factors affecting airway patency after temporary stent placement. AJR Am J Roentgenol 2007;188:1033-8. [Crossref] [PubMed]
- Conacher ID. Anaesthesia and tracheobronchial stenting for central airway obstruction in adults. Br J Anaesth 2003;90:367-74. [Crossref] [PubMed]
- Oki M, Saka H. New dedicated bifurcated silicone stent placement for stenosis around the primary right carina. Chest 2013;144:450-5. [Crossref] [PubMed]
- George TJ, Knudsen KP, Sodha NR, et al. Respiratory support with venovenous extracorporeal membrane oxygenation during stenting of tracheobronchomalacia. Ann Thorac Surg 2012;94:1736-7. [Crossref] [PubMed]
- Ko M, dos Santos PR, Machuca TN, et al. Use of single-cannula venous-venous extracorporeal life support in the management of life-threatening airway obstruction. Ann Thorac Surg 2015;99:e63-5. [Crossref] [PubMed]
- Wang L, Xu XP, Zhan H, et al. Application of ECMO to the treatment of benign double tracheoesophageal fistula: report of a case. Ann Thorac Cardiovasc Surg 2014;20:423-6. [Crossref] [PubMed]
- Gourdin M, Dransart C, Delaunois L, et al. Use of venovenous extracorporeal membrane oxygenation under regional anesthesia for a high-risk rigid bronchoscopy. J Cardiothorac Vasc Anesth 2012;26:465-7. [Crossref] [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Sidebotham D, Allen SJ, McGeorge A, et al. Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth 2012;26:893-909. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Marasco SF, Lukas G, McDonald M, et al. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ 2008;17:S41-7. [Crossref] [PubMed]
- Hong Y, Jo KW, Lyu J, et al. Use of venovenous extracorporeal membrane oxygenation in central airway obstruction to facilitate interventions leading to definitive airway security. J Crit Care 2013;28:669-74. [Crossref] [PubMed]
- Hong SH, Moon YE, Lee SR, et al. Anesthetic management for the insertion of a self-expandable metallic tracheal stent under venovenous extracorporeal membrane oxygenation. Korean J Anesthesiol 2012;63:569-70. [Crossref] [PubMed]
- Langham MR Jr, Kays DW, Beierle EA, et al. Expanded application of extracorporeal membrane oxygenation in a pediatric surgery practice. Ann Surg 2003;237:766-72; discussion 772-4. [Crossref]
- Hugh-Jones P, Lambert AV. A simple standard exercise test and its use for measuring exertion dyspnoea. Br Med J 1952;1:65-71. [Crossref] [PubMed]
- Finlayson GN, Brodsky JB. Anesthetic considerations for airway stenting in adult patients. Anesthesiol Clin 2008;26:281-91. [Crossref] [PubMed]
- Pathak V, Welsby I, Mahmood K, et al. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann Am Thorac Soc 2014;11:628-34. [Crossref] [PubMed]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators1, Davies A, Jones D, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95.
- Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166-76. [PubMed]
- Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42. [Crossref] [PubMed]
- Semaan R, Yarmus L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J Thorac Dis 2015;7:S352-62. [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]