Results of quantitative chest-CT in chronic pulmonary graft-vs.-host disease (cGvHD) 3 years after allogeneic stem cell transplantation
Introduction
Patients undergoing allogeneic stem cell transplantation (allo-SCT) are often confronted with various pulmonary complications that are usually subdivided into infectious and non-infectious as well as in early and late phase considering the time point of transplantation (1). In 40–60%, significant clinical affection with respiratory impairment can be observed (2,3). The major late post-transplantation complication is chronic pulmonary graft-vs.-host disease (cGvHD). It is one of the most frequent non-infectious late-onset complications which benefits from immunosuppressive therapy (4). Different subgroups have been described depending on clinical, radiological, histopathological findings, as well as pulmonary function tests (PFT). The most cited classification has been proposed by Palmas et al., who subdivided cGvHD into bronchiolitis obliterans (BO), BO with organizing pneumonia (BOOP), diffuse alveolar damage (DAD), lymphocytic interstitial pneumonia and interstitial pneumonia (5). In different publications, BO is equalized to an end-stage form of cGvHD (5). Current guidelines define chronic in contrast to acute types of GvHD by the absence of an acute course of the disease (6). BO as the dominant manifestation of cGVHD is defined as a reduced forced expiratory volume in 1 s (FEV1) (<75% of the predicted value), reduced relation of FEV1/vital capacity (VC) (<5–7 percentile of predicted value), evidence of air-trapping or small airway thickening on chest-CT and the absence of opportunistic pulmonary infections (6). In patients with already known cGvHD, the evidence of air-trapping or small airway thickening is no longer necessary. Whereas documentation of air-trapping requires generally the use of expiratory chest-CT measurements, the presence of bronchial wall thickening and luminal dilatation may be discrete or absent in the early stages of BO. Finally, the diagnosis of obstructive disease is knowingly the domain of expiratory tests both for PFT and chest-CT. Nevertheless, end-inspiratory chest-CT studies are practiced frequently in this patient group after allo-SCT mainly for exclusion of pulmonary infection and they are more accurate for detection of emphysema-equivalent lung parenchymal changes representing advancing pulmonary tissue destruction. As quantification of lung volume and density (attenuation) is now available as a post-processing tool for most CT-scanners this additionally gained information might be helpful for diagnosis of cGvHD.
Hence, the purpose of this study was to evaluate the role of end-inspiratory chest-CT data quantification in the early diagnosis of BO by comparison with established PFT changes occurring on average three years after allo-SCT.
Methods
Patient characteristics
Between September 2004 and July 2014, we retrospectively analysed 26 patients with chronic pulmonary GvHD (19 males, 7 females; mean age, 49.29±15.89; range, 19–72 years) at our institution that have been all examined by means of chest-CT and presented also with current PFT-data for methodical comparison. Indications for these examinations were respiratory complaints of unknown origin.
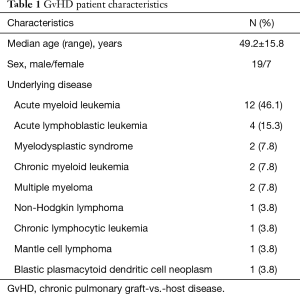
The local ethics board was informed and gave its written approval to this retrospective study, Local ethic aboard waived informed consent fort his retrospective study (number of ethic votum 237/2015R). The underlying diseases of the cGvHD patients were: acute myeloid leukemia (n=12), acute lymphoblastic leukemia (n=4), myelodysplastic syndrome (n=2), chronic myeloid leukemia (n=2), multiple myeloma (n=2), non-Hodgkin lymphoma (n=1), chronic lymphocytic leukemia (n=1) and mantle cell lymphoma (n=1) and blastic plasmacytoid dendritic cell neoplasm (n=1). All underlying diseases are tabulated on Table 1.
Full table
All patients underwent a chest-CT in the pre-transplantation (before allo-SCT) setting. The mean age at time point of transplantation was 46.89±15.29. The time point of documentation of pulmonary cGvHD was 2.99±3.21 years after allo-SCT at a mean age of 49.29±15.89. The reason for the chosen control interval was that cGVHD is mostly an asymptomatic, insidious disease occurring within the first 2 years of SCT with clinical symptoms expected first in the subsequent time.
All our patients developed respiratory symptoms like chronic cough and exertional dyspnoea within the first three years after allo-SCT.
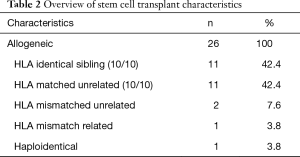
Stem cell transplant characteristics are tabulated on Table 2.
Full table
All patients received in the post-transplant setting different medications for GvHD- prophylaxis: 12 patients received mycophenolate-mofetil (MMF) (46.1%), 13 received tacrolimus (50.0%), 10 methotrexate (38.4%), 6 anti-thymocyte-globulin (ATG) (23.0%) and 7 cyclosporine (26.9%).
Clinically, a grade I cGvHD was diagnosed in 6 patients (23.0%), a grade II cGvHD was found in 2 patients (7.6%), a grade III cGvHD in 3 patients (11.5%) and a grade IV cGvHD in 2 patients (7.6%) documented either by manifestations of skin, liver, lung (42.3%) or gastrointestinal tract.
The grade of BO in our patients was additionally scored in three stages based on the FEV1%pred: mild (60–79%), moderate (40–59%) and severe (39% or less) (6). In our cohort 13 patients (50.0%) had mild, 7 patients (26.9%) had moderate and 6 patients (23.1%) had severe form of BO based on this classification. Four patients received a biopsy of the lung because of rapidly deteriorating respiratory function and evaluation for potential lung transplantation. In the other patients, a biopsy was abandoned because of their clinical status.
High resolution CT-technique
For monitoring the disease course, repeated CT examinations (56 CT-examinations containing 20 baseline-CT and 26 follow-up-CTs) were performed for the cGvHD patients.
CT-examinations were performed using helical CT-scanner scanner (Somatom Plus 4, Somatom 16, Somatom sensation 64, Somatom Definition AS+, Siemens Medical Systems, Erlangen, Germany) using a 250–330 mm field of view, a 512×512 reconstruction matrix, 120 kV, 100–150 effective mAs and a tube rotation time of 0.5/0.3 ms. No intravenous contrast-material was applied. A single spiral acquisition was obtained from the apex to the base of the lungs during one breath-hold at suspended end-inspiratory volume. Examinations were performed with patients in the supine position. Every CT scan was reconstructed at 3 mm slices with a sharp reconstruction algorithm (filter, B70) and 1 mm reconstruction increment for visual assessment and quantification (filter, B31f).
Imaging analysis
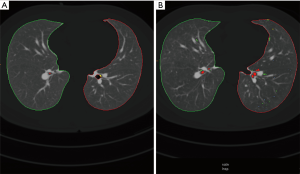
All scans were viewed at standard mediastinal windows (level, 35 HU; width, 450 HU) and lung windows (level, −700 HU; width, 1,500 HU). All examinations were viewed by one reader (MH) with 20-year experience in reading chest-CT. All patients received a quantitative imaging analysis of the lung parenchyma with the Syngo CT Pulmo 3D software (Siemens Healthcare). A CT densitometry is possible with this software (7-10). For each side, separated lung volumes and lung densities were measured. Both the percentages of lung volumes below −950 HU [low attenuation values (LAV)] and over −250 HU [high attenuation values (HAV)] were computed. Additional areas of emphysema were analysed based on their different volumes, which is called cluster analysis. Areas were classified into four subgroups based on their size (class 1 =2 mm3; class 2 =8 mm3; class 3 =65 mm3; class 4 =187 mm3). An example of cluster coded analysis (color coded display of emphysema equivalent attenuation values) is given on Figure 1.
Standard of reference
All patients underwent PFTs according to the European Respiratory society guidelines before and after intervention (11). A Masterscreen Body (CareFusion GmbH, Hoechberg, Germany) were used for the PFT before the stem cell transplantation, but also in follow up. Following PFT-parameters were assessed: VC, FEV1, single-breath diffusion capacity for carbon monoxide (DLCOcSB), single-breath diffusing capacity for carbon monoxide divided by the alveolar volume (DLCOcSB/VA) and percentage of the measured values to the predicted values (Pre).
Statistics
All results are expressed as average with standard deviation. Statistical analysis was performed using dedicated software [IBM SPSS 16.0 (SPSS), Armonk, USA]. The Kolmogorov-Smirnov test was used for the normality test including Lilliefors significance correction. Wilcoxon signed rank test was used for significance testing concerning total lung volume. Paired t-test was used for significance testing MLD, LAV, HAV and cluster analysis before and after stem cell transplantation. Paired t-test was used for significance testing concerning PFT-parameters before and after stem cell transplantation. Pearson’s r coefficients were determined for correlation analysis between total lung volumes with MLD, LAV and HAV. A value of P<0.05 was considered significant.
Results
Patient’s data
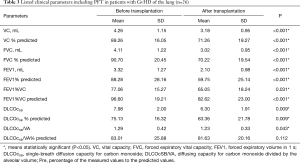
The clinical parameters of cGvHD-patients before and after stem cell are shown on Table 3.
Full table
Lung volumes and densities in cGvHD
Lung volumes at end-inspiration in cGvHD patients were 5,862.64±1,472.70 mL before stem cell transplantation and 5,934.0±1,169.18 mL after transplantation (P=0.317).
Mean end-inspiratory MLD values in cGvHD in the pre-transplantation setting were −842.29±24.29 HU. MLD values decreased after allogeneic-SCT from −823.30±38.01 to −842.29±24.29 HU; however, this did not reach statistical significance (P=0.26).
Accordingly, LAV values in cGvHD patients were 2.58±4.38 before stem cell transplantation. After stem cell transplantation, no significant differences in LAV were measured (3.53±8.77 vs. 2.58±4.38; P=0.182).
HAV values in cGvHD patients were 1.24±0.66. No significant differences in HAV were measured (1.2±0.49 vs. 1.125±0.56 HU) after stem cell transplantation, however a tendency to a decrease was registered (P=0.238).
Cluster analysis
The cluster analysis (color coded display of emphysema equivalent attenuation values depending on their size) revealed no significant differences in the values of cGvHD patients before and after stem cell transplantation. Only the cumulative values (class 1–class 4) for the lung parts showed a tendency of an increase of the values (P=0.105 left lung; P=0.108 right lung which represents the overall LAV values.
Between the different BO grade I–III subgroups, there was a significant difference between the HAV (P=0.03) which declines with higher group-score; however no differences between lung volumes, LAV or mean lung density were observed.
Correlation of CTD with PFT
Absolute differences in forced expiratory vital capacity (FVC) before and after stem cell transplantation showed positive correlations with changes of LAV (r=0.649, P=0.031). Predicted VC correlated accordingly positive with changes of LAV (r=0.771, P=0.005). There was no statistically significant correlation between LAV and DLCOcSB, however a tendency towards a growing negative correlation after transplantation (after transplantation, r=−0.354, P=0.115).
There was a correlation between the absolute difference of FEV1 and DLCOcSB measured in each patient (r=0.64, P=0.14) before and after stem cell transplantation.
Between the different degrees of BO (grade I–III), gender and underlying disease there were no statistical significant differences or correlations.
Discussion
The pathophysiological mechanism in chronic pulmonary GvHD is obstruction caused by progressive fibroproliferation within the terminal bronchioles in the lung leading to stenosis and obliteration of airway lumens (12). Accordingly, the diagnostic criteria for lung GvHD include both clinical, imaging as well as pulmonary functional test parameter that consider in the first line changes related to secondary bronchial obstruction (13). Airflow obstruction may lead if untreated rapidly to irreversible lung damage with a 5-year survival rate as low as 13% (14). Increased resistance to expiratory airflow causes with time parenchymal destruction with decrease of oxygen exchange area in the lung and restriction. Therefore, anticipation of cGvHD-related pulmonary changes is mandatory for preventing rapid decline in lung function and for institution of adequate therapy. Previous studies dealing with this post-transplantation complication used mainly CT-morphological criteria like the presence and extent of air-trapping and accompanying expiratory mosaic pattern, bronchial wall thickening (15,16) whereas a few also used novel lung density quantification software (17). Taking in consideration that many of these patients will develop at time different pulmonary complications mainly due to ongoing GvHD prophylaxis with immunosuppressive drugs, additional quantification of lung parenchymal volume and density on usual end-inspiratory chest-CTs could help to early detect cGvHD-related changes. Based on this assumption, we set out to retrospectively quantify lung parenchymal changes in patients fulfilling cGvHD (obstructive bronchiolitis) criteria that became symptomatic, in mean three years after allo-SCT (6). Our patients presented all with pulmonary obstructive disease occurring de novo in this given clinical setting confirmed by significant reduction of FEV1s, increase in RV accompanied by a not significant decrease in DLCOcSB. Interestingly, the total lung capacity (TLC) did not change significantly at time. According to our results, lung volumes at end-inspiration in cGvHD patients slightly increased after transplantation to 5,862.64±1,472.70 vs. 5,934.0±1,169.18 mL (P=0.317). Correspondingly, the average end-inspiratory density (MLD) values decreased after allogeneic-SCT from −823.30±38.01 to −842.29±24.29 HU; however, they did not reach statistical significance. LAV values increased after stem cell transplantation from 2.58±4.38 to 3.53±8.77, but even these changes did not reach statistical significance. Notably, MLD, LAV and lung volume differences between pre- and post-transplantation phases proved also independent on the degree of BO (grade I–III). This is in support of obstructive vs. destructive parenchymal changes as the latter are expected to more markedly impact lung density (MLD and LAV). Statistically, the absolute differences in FVC before and after stem cell transplantation showed positive correlations with changes in LAV (r=0.649) whereas the predicted VC correlated positive also with LAV (r=0.771).
Little is known about the course of cGvHD in the first years after allo-SCT. Longitudinal studies have stated that there is first a decline in airflow, lung volumes and diffusing capacity up to 6 months after transplantation followed by partial recovery by 1–2 years and normal airflow between 2–13 years (18-20). Others have reported either no obstruction, but a low incidence of restriction (21) or no obstruction coupled by a higher incidence of restriction (22). In our cohort, lung volume increased after allo-SCT at end-inspiration accompanied expectedly by a decrease in MLD and an increase in LAV. However, the magnitude of these changes proved not significant in our cohort, three years after allo-SCT. Notably, changes in diffusing capacity proved modest suggesting that obstruction is the leading mechanism in respiratory decline in cGvHD at this time point. This finding matches with the modest degree of emphysema equivalent (<−950 HU) areas (clusters) measured at CT-densitometry (CTD).
This study has some limitations. First, it is retrospective in character. Second, not all patients receiving repeated chest-CT in the post-transplantation clinical setting underwent also PFT, so we could not include all patients experiencing this pathology in the current evaluation. Third, we did not perform additional expiratory series in order to document obstruction by means of CT-lung parenchyma quantification.
CT-quantification at end-inspiratory phase obtained in cGvHD patients three years after allo-SCT shows only slight pathological changes (increase in lung volume and decrease in lung parenchymal attenuation) compatible with airflow obstruction. However, this technique enables exclusion of relevant parenchymal destruction (emphysema-like lung density) at this time.
Acknowledgements
The authors thank Siemens Healthcare for technical support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Song I, Yi CA, Han J, et al. CT findings of late-onset noninfectious pulmonary complications in patients with pathologically proven graft-versus-host disease after allogeneic stem cell transplant. AJR Am J Roentgenol 2012;199:581-7. [Crossref] [PubMed]
- Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest 1996;109:1066-77. [Crossref] [PubMed]
- Chan CK, Hyland RH, Hutcheon MA. Pulmonary complications following bone marrow transplantation. Clin Chest Med 1990;11:323-32. [PubMed]
- Duncker C, Dohr D, Harsdorf S, et al. Non-infectious lung complications are closely associated with chronic graft-versus-host disease: a single center study of incidence, risk factors and outcome. Bone Marrow Transplant 2000;25:1263-8. [Crossref] [PubMed]
- Palmas A, Tefferi A, Myers JL, et al. Late-onset noninfectious pulmonary complications after allogeneic bone marrow transplantation. Br J Haematol 1998;100:680-7. [Crossref] [PubMed]
- Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945-56. [Crossref] [PubMed]
- Blechschmidt RA, Werthschutzky R, Lorcher U. Automated CT image evaluation of the lung: a morphology-based concept. IEEE Trans Med Imaging 2001;20:434-42. [Crossref] [PubMed]
- Bankier AA, De Maertelaer V, Keyzer C. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999;211:851-8. [Crossref] [PubMed]
- McGuinness G, Naidich DP, Leitman BS. Bronchiectasis: CT evaluation. AJR Am J Roentgenol 1993;160:253-9. [Crossref] [PubMed]
- Brillet PY, Fetita CI, Saragaglia A, et al. Investigation of airways using MDCT for visual and quantitative assessment in COPD patients. Int J Chron Obstruct Pulmon Dis 2008;3:97-107. [Crossref] [PubMed]
- Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153-61. [Crossref] [PubMed]
- Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17:1072-8. [Crossref] [PubMed]
- Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:208-14. [Crossref] [PubMed]
- Yoshihara S, Yanik G, Cooke KR. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007;13:749-59. [Crossref] [PubMed]
- Padley SP, Adler BD, Hansell DM. Bronchiolitis obliterans: high resolution CT findings and correlation with pulmonary function tests. Clin Radiol 1993;47:236-40. [Crossref] [PubMed]
- Gunn ML, Godwin JD, Kanne JP. High-resolution CT findings of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. J Thorac Imaging 2008;23:244-50. [Crossref] [PubMed]
- Mocelin H, Bueno G, Irion K, et al. CT densitovolumetry in children with obliterative bronchiolitis: correlation with clinical scores and pulmonary function test results. J Bras Pneumol 2013;39:701-10. [Crossref] [PubMed]
- Bruno B, Souillet G, Bertrand Y. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant 2004;34:143-7. [Crossref] [PubMed]
- Kaplan EB, Wodell RA, Wilmott RW. Late effects of bone marrow transplantation on pulmonary function in children. Bone Marrow Transplant 1994;14:613-21. [PubMed]
- Nysom K, Holm K, Hesse B, et al. Lung function after allogeneic bone marrow transplantation for leukaemia or lymphoma. Arch Dis Child 1996;74:432-6. [Crossref] [PubMed]
- Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001;120:1900-6. [Crossref] [PubMed]
- Nenadov Beck M, Meresse V, Hartmann O. Long-term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant 1995;16:771-5. [PubMed]


