A female patient with recurrent lung lesion and hilar lymphadenopathy
Joint hospital grand rounds have been held in Guangzhou Institute of Respiratory Disease (GIRD) routinely every week for more than 10 years. Recently, more and more hospitals are taking part in our joint rounds through real-time video communication. Such events are broadcast live to healthcare providers from up to 500 hospitals in China. This paper presents the joint grand round between GIRD and Firestone Institute for Respiratory Health (FIRH) on October 18th, 2016. Specialists from FIRH, St Joseph’s Healthcare and McMaster University, Hamilton, ON, Canada included Prof. Paul M. O’Byrne, MD., PhD.; Prof. Martin Kolb, MD., PhD.; Prof. Gerard Cox, MD., PhD.; Prof. Andrew Mclvor, MD., PhD.; and Prof. Hongyu Wang, MD., PhD. Specialists from GIRD included Prof. Nanshan Zhong, MD., PhD.; Prof. Rongchang Chen, MD., PhD.; Prof. Shiyue Li, MD., PhD.; Prof. Linling Cheng, MD., PhD.; Prof. Qingsi Zeng (radiologist); and Prof. Yinyin Gu (pathologist). The details of the grand round are as follows.
Case presentation
Dr. Linling Cheng (pulmonologist at GIRD):
A 42-year-old Chinese female complaining of recurrent cough and lung opacity was admitted to the GIRD, the First Affiliated Hospital of Guangzhou Medical University between October 10, 2016 and October 13, 2016. She had no history of other medical illness. She was a teacher and had no history of tobacco or alcohol abuse.
She started to cough after a cold 3 years ago, without phlegm, fever, hemoptysis, shortness of breath, chest pain, night sweats, fatigue or weight loss. She presented to Guangzhou Panyu Central Hospital. Computed tomography (CT) of the chest showed bilateral pneumonia and pleural effusion. Blood analysis showed normal white blood cell count and neutrophil count. Procalcitonin was 0.11 ng/mL. The symptoms persisted after treatment with moxifloxacin and antitussive for 2 weeks. Another CT scan showed no obvious resolution of the opacities, but a new small cavity with thick walls was noted in the right lower lobe. Transbronchial lung biopsy (TBLB) was done in the right middle lobe (RML), which was inconclusive. Then, she presented to GIRD on August 29, 2013. Subsequent chest CT scan revealed scattered opacities in both lungs, enlarged pretracheal and right hilar lymph nodes, and pleural effusion on the right side (Figure 1A,B). Review of lung biopsy (RML) from Panyu Central Hospital showed: red blood cells and foamy cells in alveolar lumen, with few organizing lesions; multiple tuberculoid nodules in interstitial tissues, without caseous necrosis or lymphocyte infiltration; special staining: anti-acid (−), periodic acid-silver metheramine (−), Ag (−), PAS (−); immunohistochemistry: ACE (−), NGF (+), NT3 (−); ultimately, the report stated “tuberculosis is considered”. Then the patient was referred to Guangzhou Chest Hospital, which specialized in diagnosis and treatment of pulmonary tuberculosis (TB), and she received standard anti-TB treatment for 1 year. However, she had recurrent cough and the opacities on chest CT persisted.
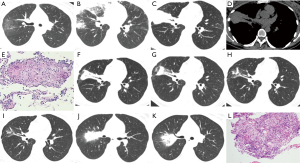
The patient re-visited GIRD in December 2014. CT scan showed that some of the scattered opacities had increased while others decreased in size; there were no significant changes in the enlarged hilar and pretracheal lymph nodes; the pleural effusion had disappeared (Figure 1C,D). The patient was re-hospitalized in GIRD on December 5th, 2014. Blood analysis showed normal white blood cell count again. Procalcitonin was normal. Results of acid-fast bacilli in sputum were negative on three consecutive smears. TBLB in left upper lobe (LUL) revealed multiple granulomatous nodules with clear borders; epithelioid cells and polykaryocytes were visible but there was no caseous necrosis within the nodules; special staining: anti-acid (−), periodic acid-silver metheramine (−), Ag (−), PAS (−), suggesting non-necrotic granulomatous disease (Figure 1E). This was interpreted as “sarcoidosis with tuberculosis as differential diagnosis” by the pathologist. Therefore, sarcoidosis was diagnosed after considering both the clinical and pathological data. Prednisone at a dose of 0.5 mg/kg/d was prescribed. The cough and phlegm disappeared with therapy. Subsequent chest CT scans between January and October 2015 showed obvious resolution on RML, right upper lobe (RUL) anterior segment, and LUL lingula segment (Figure 1F-I). The dose of prednisone was gradually reduced to 15 mg once every other day for 6 months.
Her cough relapsed with a small amount of white sticky phlegm in June 2016, about 3 months after reduction of prednisone to low dose on alternate days. She had no symptoms of fever, hemoptysis, weight loss, shortness of breath, joint pain, skin rash or skin itching. Phlegm decreased after initiation of traditional Chinese medicine therapy (details are not available). She visited our clinic on August 23rd. Chest CT scan indicated new opacities in the RUL (Figure 1J,K). Pulmonary function testing showed a mild restrictive ventilatory defect and moderate diffusion impairment. She slept well.
Physical examination showed a temperature of 36.7 °C, pulse 97 bpm, respiratory rate 20 bpm, blood pressure 94/55 mmHg, and oxygen saturation 100% on room air. She was alert, oriented and appeared normal. No positive signs were noted on skin, no superficial lymph nodes. Thoracic expansion and tactile fremitus were symmetric bilaterally. Breath sounds were normal, without wheezes or rales. The remainder of the examination was normal.
Blood analysis on August 27th, 2013, December 5th, 2014, and October 12th, 2016 showed normal white blood cell counts and neutrophil counts. Procalcitonin was slightly elevated at 0.11ng/mL on August 27th, 2013; however, it normalized in December 2014 and September 2016. Sputum acid-fast bacilli tests were negative on three consecutive smears during her hospitalization in October 2016. Sputum samples collected during bronchoscopy showed that TB-DNA was negative on three occasions (December 2014, September 2016, and October 2016). Testing for acid-fast bacilli in the smear of lung brush samples also was negative on three occasions (December 2014, September 2016, and October 2016). Testing for antinuclear antibody was negative. p-ANCA and c-ANCA were negative. Other anti-nuclear antibody spectrums also were negative. Levels of immunoglobulin (Ig) G, IgM and IgA were normal. TBLB was re-performed in the upper lobe of right lung on October 12th, 2016 and biopsy was reviewed by the pathologist, Prof. YinyinGu.
The key points to be discussed included: first, was the empirical anti-TB treatment necessary at the onset of illness when the diagnosis was not clear? What was the optimal duration for empirical anti-TB treatment? Second, was medication intervention necessary when the diagnosis of sarcoidosis was made? Third, what caused this new pulmonary lesion? Was it recurrent sarcoidosis or concurrent infection by an unknown pathogen?
Prof. Nanshan Zhong (Grand round moderator, pulmonologist at GIRD):
Thank you for your presentation, Prof. Cheng. I supposed that we had difficulty in making the diagnosis at the moment. At the onset of illness, the patient was suspected to have tuberculosis as there was an opacity with cavity in the right lower lobe and the biopsy in the RML showed multiple tuberculoid nodules, so anti-TB treatment was prescribed for one year. However, the opacities didn’t resolve and her cough recurred. Another biopsy performed in the LUL showed multiple granulomatous nodules without caseous necrosis, hence, sarcoidosis was suspected and prednisone was prescribed. Her cough improved and the opacities resolved gradually on follow-up visits. Later, a new shadow appeared in the RUL, when prednisone was tapered down to 15 mg every other day. A new biopsy was conducted; however, the diagnosis was still uncertain. Now, I would like to invite radiologist Prof. Zeng to review the characteristics of CT scan and pathologist Prof. Gu to review the biopsy findings on the lesion in the RUL.
Prof. Qingsi Zeng (radiologist at GIRD):
The patient underwent chest CT 8 times in our hospital between August 2013 and October 2016. The earliest CT scan in August 2013 showed multiple consolidations in both lungs, with unclear borders in the lesions in the RUL and RML. There were some enlarged lymph nodes, in bilateral hilar areas and the mediastinum. The largest lymph node was located below the carinal, with a size 9 mm × 20 mm. The CT scan in December 2014 showed that the density of consolidation in RML increased with a clear border, and consolidation in other lobes decreased. In the follow-up CT scans between January 2015 and October 2015, the consolidation gradually decreased and the lesions on both sides resolved gradually. However, in August 2016, opacity in the RUL lesion and the lymph node in the RUL enlarged. Based on the findings on CT scan, including the homogeneous lesion without cavitation or necrosis, we considered the diagnosis was more likely to be sarcoidosis. However, the possibility of TB couldn’t be excluded.
Prof. Yinyin Gu (pathologist at GIRD):
Biopsy of the upper lobe of right lung on October 12th, 2016 showed the shedding of mucosa and submucosa edema, with many infiltrating lymphocytes and two granulomatous nodules on the border. Borders of both granulomatous nodules were not clear. No obvious necrosis was noted. There were many lymphocytes infiltrating the nodules. The type of polykaryocytes was mainly Langhans’ giant cells. Special staining: anti-acid (−), periodic acid-silver metheramine (−), PAS (−). The biopsy in the upper lobe of right lung suggested non-necrotic granulomatous disease; hence, sarcoidosis could be considered if tuberculosis and non-tuberculous mycobacteria were excluded clinically (Figure 1L). We should consider sarcoidosis combined with lymphoproliferative diseases because there were many lymphocytes infiltration in the nodules.
Diagnosis discussion
Prof. Nanshan Zhong:
Pathology results a week ago showed granulomatous lesion that were not typical of sarcoidosis, and obvious lymphocytic infiltration. The patient had a poor response to anti-TB treatment for 1-year and had a very good response to corticosteroid treatment. But the lesions in the RUL on the CT scan enlarged when the dose of corticosteroid was tapered down to 15 mg every other day, indicating relapse. Hence, granulomatous disease was considered, and I doubted whether the patient had coincident lymphoproliferative diseases.
Prof. O’Byrne (pulmonologist at FIRH):
While granulomatous inflammation and lymphocytic infiltration are present, the exact diagnosis is unclear at the moment.
Prof. Gerard Cox (pulmonologist at FIRH):
Let’s go back to the beginning when the radiology report says there was a cavity. I’m not sure that we have seen a cavity yet. I wonder if the appearance on the CT at that time might have a different explanation, which would change our ideas quite a lot. Because of infection was considered, the patient was treated with anti-TB therapy for a year. TB should be always kept on mind when granulomatous lesion was found, particularly when infection was considered. In this case, whether or not there were cavity inside the lesion is very important for the consideration of diagnosis. It is interesting that there were few symptoms of infection and no infective organisms were found despite rigorous detections have been done with many times of sputum testing and lung biopsy in several occasions. I took that evidences to be quite strong against the illness being due to ongoing infection. Another thing that I notice is the lymphocytic infiltration of tissues. That might explain the good response to steroid. I, like Dr. O’Byrne, wonder if her illness is lymphocytic granulomatosis.
When we think about sarcoidosis, we should look for disease outside of the lungs. So far everything we have seen involves only the lungs and the lymph nodes at the center of the chest. One feature that you mentioned was a subcutaneous nodule in her arm and another on her back. I think subcutaneous nodule biopsy might be considered. This could show she had a systemic disorder as opposed to just a lung disorder with reactive lymph nodes in the center of chest.
Another thing we would review is her serology. In the documents we saw, the antinuclear antibody spectrum test was negative. I wonder about ANCA—because GPA could explain the granulomatous inflammation. We have a few patients who have nodular lesions with granulomatous inflammation in the lungs. This condition would be steroid responsive.
The last thing I would like to say is when we don’t know what’s wrong with somebody, we go back to the sources of information. I think that we are probably not enough in learning the patient’s history and if there was exposure to something. I get nervous when I hear about someone had disease and received traditional Chinese medicine because I know so little about it. I didn’t know what’s involved. I don’t know if there was potential for something to cause a hypersensitivity reaction. I think the images are complete; I would not do anymore trans-bronchial biopsies because a bigger sample of tissue e.g., from surgical lung biopsy, or cryobiopsy, is needed for a more confidant diagnosis. I don’t think that doing an endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) of lymph nodes would be useful, the sample is too small and the nodes could easily be reactive. We don’t know the diagnosis and I would like tissue samples for diagnosis, before recommending any further treatments.
Prof. Nanshan Zhong:
Thank you, Dr. Cox. I’d like to answer your questions. The ANCA test showed negative, and there was no evidence showing vasculitis in this patient. The patient was not exposed to some special environments. She took some Chinese medicines, but we don’t know what the medicines were. According to the biopsy on one of the subcutaneous nodules, there was no evidence showing sarcoidosis. The patient mentioned that she still have some subcutaneous nodules. However, I didn’t find any typical subcutaneous nodule after careful physical examination, probably due to the effect of treatment. We agreed with you that sometimes TBLB or biopsies might not be enough to make a diagnosis, particular in this kind of patients. So we would prefer use mini-invasive surgical lung biopsy. A new surgical procedure has been developed by our surgical team, which enable us to perform surgical lung biopsy with a single hole or very small incision. Does anyone like to make a comment of this case?
Prof. Rongchang Chen (pulmonologist at GIRD):
The patient was still taking prednisone of 15 mg every other day. Does sarcoidosis relapse common when the patient was still on low dose of oral corticosteroid?
Prof. Martin Kolb (pulmonologist at FIRH):
Dr. Cox nicely described what we don’t know. Now we are in a good position to see that it is likely not tuberculosis because appropriate anti-TB treatment for one year didn’t change the course of the disease. So it can probably be ruled out by now. We have a granulomatous disease with lymphocytes, which responds to steroid therapy. I personally don’t feel convinced yet this is sarcoidosis. I would agree with Dr. Cox that larger pieces of tissue would help. One possibility we didn’t mention yet is organizing pneumonia. We know the finding of granulomatous disease can be pretty nonspecific. The quality of images is not good enough for me to say if there truly is cavitation. From the images we printed out, I would rather call it reverse halo sign, which is more commonly found in organizing pneumonia and sometimes in TB. So organizing pneumonia would be something I would consider. From the treatment perspective, and considering that this lady doesn’t want any more biopsies, it is clear that she responded to steroids, and relapsed after reduction of the dose to alternate days. If the symptom relapsed, one might need to increase prednisone again and keep her at a slightly higher dose to keep the disease in remission. Daily doses of 10–15 mg would be an acceptable approach even if you don’t have a firm diagnosis.
Prof. Nanshan Zhong:
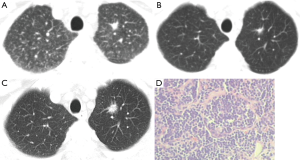
Dr. Cox had mentioned about some kind of granuloma disease and Dr. Kolb had mentioned about granulomatous lymphocytic inflammation. I agreed with both of your opinions. I would like to introduce one of my cases to you, which is similar to the present case, but I am not quite sure. This is the case with sarcoidosis combined with pulmonary mucosa-associated lymphoid tissue lymphoma (PMALTL). The patient visited our hospital for consultation in 2009. Chest CT scan showed diffuse nodules, and there was a nodule in the LUL, which was similar to the patient with nodule shadow (Figure 2A). The figures showed multiple lesions in the whole lung, then this patient underwent biopsy, with which the patient was considered with sarcoidosis. With the treatment of corticosteroid for one month, the nodule in LUL still existed but the nodules became much less and lesions in other fields ultimately disappeared (Figure 2B). However, the nodule in LUL was getting larger 3 years later (Figure 2C). We were worried about that, so we performed upper left lobectomy in 2012. During the surgery, we can see the same thing that biological change in the case we presented to you. Biopsy showed granuloma, but the borderlines were not that clear. So actually diagnosed by Dr. Gu, the case had PMALTL combined with sarcoidosis (Figure 2D). We supposed the patient with sarcoidosis combined with PMALTL. Follow up CT scan in 2013, 2015 and 2016 showed there is no relapse. Just now, Dr. Cox mentioned about granulomatous lymphatic inflammation. We suppose that this patient might have the same situation, but I’m not sure. My comment is that if the lesion is still getting larger and larger, should we do another biopsy, or even to remove that lesion? Maybe there would be another diagnosis in addition to sarcoidosis.
Final summarization
Prof. Nanshan Zhong:
Ultimately, let me make a summary. Dr. Cox and Dr. Kolb think the patient may have a granulomatous disease. Our opinion is that we should pay attention to the RUL lesion. The lesion of the case discussed is similar to the PMALTL patient. Sarcoidosis couldn’t explain the whole course of the patient in discussion. The patient’s condition is OK now. But the biopsy had not confirmed the feature of sarcoidosis. Operation should be considered if the lesion gets larger. Thank you for your attention. Details of subsequent treatment and follow up would be presented at later issue.
Acknowledgements
All authors are gratitude to AstraZeneca for supporting this real-time video joint grand round. We thank Dr. Qian Han for her translating the result of biopsy into English during the grand round, and we thank Dr. Zheng Zhu for providing the images and slides of the patient with PMALTL.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.


