Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer
Introduction
Neoadjuvant therapy (preoperative chemotherapy, targeted therapy and radiotherapy, either alone or in combination) can significantly improve the resection rate for patients with locally advanced non-small cell lung cancer (NSCLC) and can prolong their survival (1). However, there is also concern that neoadjuvant therapy may promote pleural adhesion and vascular fragility, which is unfavorable to the anatomy and hemostasis and may increase the postoperative complication rate and perioperative mortality (2). The toxic effects of neoadjuvant therapy may undermine the constitution of patients and affect the patients’ ability to heal, making it difficult for patients to tolerate the conventional open thoracotomy (3), and the neoadjuvant therapy thus is not conducive to patients who were planed to undergo the surgical resection.
Compared with the conventional open thoracotomy, complete video-assisted thoracoscopic surgery (c-VATS) is less invasive and allows a faster recovery among patients (4,5). Theoretically, certain patients with poor physical fitness can still receive c-VATS after neoadjuvant therapy, even if they are unable to tolerate open thoracotomy. VATS has been performed in our center since 1994. To date, we have an accumulated experience of more than 1,000 cases of VATS (c-VATS, Hybrid VATS) lobectomies (6-10), including more than 150 cases of VATS sleeve lobectomies (7). Since 2006, we have attempted to perform c-VATS in locally advanced NSCLC patients after neoadjuvant therapy. To the best of our knowledge, there was no report on the feasibility and clinical effectiveness of c-VATS following neoadjuvant therapy for the treatment of locally advanced NSCLC. In this study, we explored the feasibility of c-VATS following neoadjuvant therapy (chemotherapy, targeted therapy and radiotherapy, either alone or in combination) for the treatment of patients with locally advanced NSCLC, and its perioperative complications.
Patients and methods
Patients
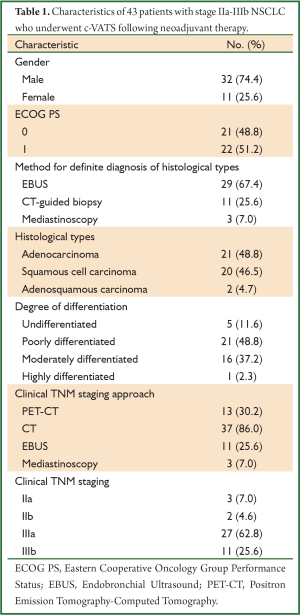
A total of 43 IIA-IIIB NSCLC patients, who were treated in our center from January 2006 to March 2012, were included in this study. All patients were stratified based on their ECOG performance status [0-1]. These patients completed preoperative chemotherapy, targeted therapy, and radiotherapy (alone or in combination) and underwent c-VATS. There were 32 men and 11 women in the study, aged 35-76 years (mean: 56.30±10.15 years; median: 57 years).
All cases were histopathologically diagnosed as NSCLC preoperatively. The histological diagnosis was confirmed by fibrobronchoscopy, EBUS and CT-guided percutaneous needle biopsy. The diagnoses included adenocarcinoma (n=21, 48.8%), squamous cell carcinoma (n=20, 46.5%) and adenosquamous carcinoma (n=2, 4.7%). The types included undifferentiated carcinoma (n=5, 11.6%), poorly differentiated carcinoma (n=21, 48.8%), moderately differentiated carcinoma (n=16, 37.2%) and highly differentiated carcinoma (n=1). The clinical stages were clinically assessed and intraoperatively confirmed through PET-CT, MRI, and CT. Cases of clinical stages N2 or N3 were staged through mediastinoscopy or EBUS. TNM staging was based on the 2009 UICC staging criteria (7th edition) (11) with 27 cases of Stage IIIA (62.8%) and 11 cases of stage IIIB (25.6%) (Table 1).
Full Table
Neoadjuvant therapy
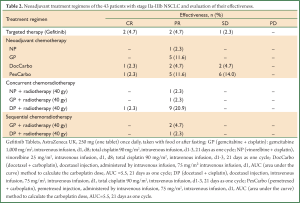
The patients were requested to select a neoadjuvant treatment regimen based on the expression of Predictive Molecular Markers, including TS (12), RRM1 (12), ERCC1 (13) and TUBB3, BRCA1, and TYMS protein, as determined by ICH and/or gene mutation test results. The neoadjuvant treatment approaches that were adopted included preoperative targeted therapy, preoperative chemotherapy, preoperative concurrent radiochemotherapy, and preoperative sequential radiochemotherapy (Table 2). Five patients positive in the EGFR gene mutation detection received gefitinib treatment. Other regimens included GP (n=8, gemcitabine + cisplatin), DocCarbo (n=6, docetaxel + carboplatin), DP (n=10, docetaxel + cisplatin), and PexCarbo (n=12, pemetrexed + carboplatin). Twenty-two patients received preoperative chemotherapy alone, 13 received concurrent radiochemotherapy, and 3 received sequential radiochemotherapy (Table 2). Patients underwent 2-3 cycles of neoadjuvant therapy. Four weeks after the end of treatment, the patient received CT scan again. If disease progression was confirmed by imaging, surgical treatment was not given; if staging down-regulation or no lesion progression was confirmed by the imaging, c-VATS was performed. The mean length of time from the end of the neoadjuvant therapy to the operation was 31.21±20.17 d (range: 3-79 d).
Full Table
C-VATS surgical techniques
Body position
The patients underwent a double-lumen endotracheal intubation under general anesthesia; in a contralateral supine position, the upper limb of the affected side was positioned on the hand bracket.
Incision selection
The observation hole was positioned at the level of the 7th or 8th intercostal space on the posterior axillary line with the main manipulative incision 3 cm to the anterior axillary line as the center, an upper lobectomy at the 4th intercostal space and a lower lobectomy at the 5th intercostal space, which allowed two surgical tools to enter or leave simultaneously. The harmonic scalpel was operated together with the suturing instrument and the aspirator. The auxiliary manipulative incision, measuring approximately 1 cm in length, was made at the same intercostal space posterior to the posterior axillary line as the observation hole for the auxiliary operation.
Surgical approaches
The surgeon stood in front of the patient, completing the procedures through the manipulative incision via the screen without using the rib distractor during the operation or operating under direct vision. The veins, arteries, and bronchia were separated anatomically, and the lymph nodes in stations 10 and 11 were dissected. The specimen bags were inserted to remove lung tissue, and the mediastinal lymph node dissection was subsequently performed again (on the left, stations 4, 5, 6, 7, 8, and 9; on the right, stations 2, 4, 7, 8, and 9). For patients whose tumor masses were closely related to blood vessels or bronchia, bronchial/vascular sleeve resection was selected as appropriate, and the patients were transferred to Hybrid VATS when necessary. The specific procedures were conducted in accord with the literature (4,11). The volume of the fluid replacement was strictly monitored intraoperatively, and the tracheal catheter was extubated in a routine manner after the operation.
Observation indicators and follow-up
The c-VATS resection rate, rate of conversion to thoracotomy, operation time, intraoperative blood loss, number of lymph nodes dissected, postoperative catheter drainage time, postoperative hospital stay, and postoperative complications (e.g., air leakage, bronchopleural fistula, and wound infection) were observed. Patients received follow-up regularly after discharge. The first follow-up was conducted postoperatively at 2-4 w. During the first three years, patients were followed up every 3-6 months and every 6-12 months thereafter. Patients’ survival was followed up via telephone, written communication, or site visit. Patients received routine outpatient examinations of a chest CT and abdominal B ultrasound. A brain MRI was conducted if necessary.
Statistical analysis
A survival analysis was conducted using the Kaplan-Meier method, and survival was calculated beginning with the point in time that point patients were diagnosed with NSCLC. Follow-up continued until May 17, 2002. P<0.05 is considered to be statistically significant. Data were entered into the database and statistically analyzed using SPSS13.0 software (SPSS, Inc, Chicago, IL).
Results
Clinical efficacy and toxic effects of neoadjuvant therapy
All 43 patients were followed up. There was no disease progression from the neoadjuvant therapy to the surgery; the postoperative histopathological stages of 25 patients were lowered, demonstrating the effectiveness of the neoadjuvant therapy; 18 patients had no changes in staging either before or after neoadjuvant therapy; 11 of these patients showed smaller lumps or lymph nodes with regard to imaging performance but did not reach PR. Among 25 patients who were responsive to the neoadjuvant therapy, one was pathologically completely response (pCR) after the operation (Table 2).
The most important adverse reactions of neoadjuvant targeted therapy were rashes and constipation. The primary adverse reactions of preoperative chemotherapy included leukopenia, nausea/vomiting, and hair loss, including grade I-II leukopenia (n=17), grade III leukopenia (n=3), grade I-II nausea and vomiting (n=8) and grade III nausea and vomiting (n=3), which were alleviated after symptomatic treatment. The common side effects of preoperative concurrent radiochemotherapy and sequential radiochemotherapy also included radiation esophagitis (n=2) and radiation pneumonia (n=5).
Surgical results and complications
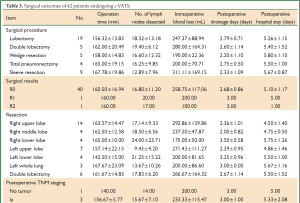
Forty-two of the 43 patients underwent successful resections with a resection rate of 97.7% (42/43). Seven patients were transferred to receive Hybrid VATS (7/42, 16.7%). The operation time was 130-180 min (mean: 160.48±16.52 min); the intraoperative blood loss was 253.57±117.08 mL; the number of lymph nodes dissected was 16.88±10.93; the postoperative drainage time was 1-7 d (mean: 2.62±0.96 d); and the postoperative hospital stay was 3-7 d (mean: 5.45±1.30 d). The surgical approaches included c-VATS lobectomy (n=28, including 9 cases of bronchial sleeve resection), c-VATS double lobectomy (n=5), c-VATS wedge resection (n=5), and c-VATS total pneumonectomy (n=4).
In terms of surgical complications, 5 patients developed postoperative complications (11.6%). Air leakage, chylothorax, wound infection, and respiratory failure were observed in five different patients. Another 67-year-old male patient died of heart failure three days after the operation. The patient had a history of preoperative hypertension and had received sequential radiochemotherapy. The patient continually suffered from radiation esophagitis, but his symptoms were remitted after symptomatic treatment. The operation went smoothly. On the second postoperative day, the patient died of sudden heart failure due to the inability to control the volume of fluid replacement. This patient represented the only perioperative death in this study (Table 3).
Full Table
Postoperative survival rate
Patients in this study were all followed up for 4-68 months (mean: 20.78±16.89 months). Eleven patients suffered from a recurrence or metastasis after the operation: skull metastasis (n=5), bone metastasis (n=3), adrenal metastasis (n=2), and recurrence (n=1). The recurrent patient underwent an R2 resection. Among the 11 patients with a recurrence or metastases, 10 died, and one simultaneously underwent gamma knife treatment whole brain radiotherapy after brain metastasis and has survived for 23 months. The median overall survival (OS) in this study was 33.0 months (95% CI, 14.7-51.4 months) with a 1-year survival rate of 94%, a 2-year survival rate of 79%, and a 3-year survival rate of 65%.
Discussion
Studies have shown that c-VATS has validated advantages in the treatment of early-stage NSCLC; it has been applied in treating locally advanced NSCLC (7,14-16). In recent years, a series of studies have suggested that surgical resection following neoadjuvant therapy for patients with locally advanced NSCLC can significantly improve the surgical resection rate and survival (1). However, neoadjuvant therapy will inevitably lead to tissue adhesion, an indistinct interface and increased vascular fragility, and it will have a definite impact on patients’ ability to heal. The incidence of surgical complications after neoadjuvant therapy has been reported to be as high as 35-43.5% (2,17). Therefore, the safety of surgical resection following neoadjuvant therapy remains a clinical concern. Theoretically, the difficulty of performing c-VATS following neoadjuvant therapy is greater than without neoadjuvant therapy. In fact, the length of the operation time is one of the commonly used indicators to evaluate the feasibility of c-VATS. The length of the operation time of c-VATS in this study was 130-180 min (mean: 160.93±16.59 min), which was consistent with times (130-168.6 min) in the previous published studies (15,18,19).
The postoperative drainage time and the length of the postoperative hospital stay can reflect the effects of surgery on patients’ ability to heal. In our current study, the postoperative drainage time was 1-7 d (mean: 2.56±0.98 d), and the postoperative hospital stay was 3-7 d (mean: 4.98±1.32 d). Tomaszek et al. (18) reported that the postoperative drainage time in their study was 1-12 d (mean: 2 d), and the postoperative hospital stay was 1-12 d (mean: 4 d). The postoperative drainage time of the majority of other c-VATS procedures are in line with our results (14,15,20).
The rate of conversion to thoracotomy is another important indicator to evaluate the safety of VATS procedures. The literature shows that the rate of c-VATS conversion to thoracotomy ranges from 0-15.7% (14). No patient was converted to conventional thoracotomy in our study, but 7 patients were converted to Hybrid VATS with a conversion rate of 16.7%. It is believed that the preoperative adjuvant therapy (especially preoperative radiotherapy) has an obvious effect on a patient’s body. In fact, this treatment can easily induce local tissue inflammation, edema and organization and thus increase the tissue’s fragility, which can cause gap fuzziness and dense adhesion, making the surgery even more difficult. In this regard, Hybrid VATS has the advantages of thoracotomy under direct vision and a large operational space and at the same time can avoid the limitations of c-VATS; therefore, this therapy can replace thoracotomy in operations including tissue isolation, anatomic lobectomy, and systematic lymph node dissection (20). Lymph node dissection can be performed on different stations in line with the standards in this study with an average dissection number of lymph nodes of 16.88±10.93, which fully meets the criteria of conventional c-VATS and thoracotomy (14,15,21).
In this study, the incidence of postoperative complications and the mortality rate were 11.9% (5/42) and 2.4% (1/42), respectively, similar to those of thoracotomy following neoadjuvant therapy [Gilligan et al. (22): 10%, 224/229] and similar to those reported for other pure c-VATS procedures [McKenna et al. (16): 15.3%; Kim et al. (23): 9.1%]. A common concern is that adhesion due to neoadjuvant therapy can often result in a long operation time, high operational risk, and a high incidence of postoperative complications. However, this phenomenon was not observed in our study, which may be due to the following factors: (I) c-VATS is minimally invasive and less painful and predisposes patients to coughing. This treatment can therefore reduce infection, atelectasis, respiratory failure, and other complications caused by poor expectoration. In our current study, the mean postoperative drainage time did not exceed 3 days, which is more conducive to postoperative expectoration and also reduces the incidence of infections of the drainage opening; (II) c-VATS has a locally magnifying ability, which is not only beneficial to the identification of intraoperative vessels and bronchia but also conducive to the detection of small bleeding spots, lung fissures and bronchial fistulas, thereby reducing the occurrence of operation-related complications; (III) The surgeon’s experience in managing complex and highly difficult procedures under c-VATS is also important to reduce the occurrence of postoperative complications and to lower the operative mortality. Our rich experience in VATS lobectomies (3) facilitated the launching of this study, which enrolled nine patients who received bronchial sleeve resection/plasty, four of whom received c-VATS (completed), 5 of whom were converted to Hybrid VATS (completed), and only one of the nine patients experienced chylothorax but without a bronchopleural fistula; (IV) The application of neoadjuvant therapy was based on gene mutation detection and drug gene (protein) test results, which are conducive to increasing the tumor response rate and reducing the damage to normal tissues. In this study, all five of the EGFR mutation-positive patients selected gefitinib therapy; TS enzyme expression-negative patients selected pemetrexed therapy (12); all of the patients without remarkable clinical significance in multi-drug gene (protein) expression selected the third-generation platinum-based chemotherapy with an overall response rate of 58.1%. These approaches can avoid poor impacts (e.g., poor target effects, strong impact on normal tissue, and impaired immunity) due to less optimized neoadjuvant therapy; (V) Because the impact of the different neoadjuvant therapy regimens on postoperative recovery differs, it is important to reduce the difficulty of the operation by selecting neoadjuvant therapy regimens based on genetic testing and drug gene (protein) detection results while reducing the proportion of preoperative radiotherapy. As shown by our study, the pre-operative application of targeted therapy drugs had a minimal impact on human tissues and typically did not cause remarkable tissue adhesion, scarring, or edema while maintaining a vascular toughness that was close to normal. On the contrary, radiotherapy often leads to breast tissue edema or adhesion, thus increasing the difficulty of the procedures. Among the 16 patients receiving preoperative radiotherapy, 6 patients (6/16, 37.5%) were converted to Hybrid VATS; among the remaining patients who did not receive radiotherapy, only one patient (1/27, 3.7%) was converted to Hybrid VATS. In addition, several scholars (17) have argued that a preoperative radiotherapy dose of >45 Gy might significantly increase the incidence of postoperative complications. This finding was validated in our current study: the incidence of postoperative complications was not high among patients receiving preoperative radiotherapy, which might be observed because the radiotherapy dose in this study was not higher than 40 Gy.
The long-term efficacy of this study was satisfactory with a 1-year survival rate of 94%, a 2-year survival rate of 79%, and a 3-year survival rate of 65%, which may be observed because c-VATS is minimally invasive. After neoadjuvant therapy, patients may still tolerate c-VATS, even with a poor constitution or impaired lung function. Therefore, more patients can receive this procedure, and the overall survival rate is also increased. Patients can recover from c-VATS faster and were able to complete postoperative adjuvant radiochemotherapy. Lymph node dissection of c-VATS is not inferior to conventional thoracotomy. The rational combination of c-VATS with neoadjuvant treatment improves the overall response rate. However, a larger sample size is warranted to validate this conclusion further.
This study investigated the impact of neoadjuvant therapy on c-VATS among NSCLC patients, but it failed to compare the findings with those of thoracotomy. Furthermore, the success of c-VATS is highly dependent on the surgeon’s experience and skills. The postoperative complications and long-term efficacy differ if the c-VATS is performed by different surgeons. Additionally, the small sample size of this study can easily cause bias. Neoadjuvant therapy for tumors has become increasingly popular (24), and in our current study, we assessed neoadjuvant treatment regimens based on the results of genetic tests and drug molecular detection and assessed the relevant indicators. However, these detection methods also resulted in a diversity of neoadjuvant treatment regimens, causing the statistical analysis to be more difficult. No definite conclusion was reached on the impact of diverse neoadjuvant regimens on the perioperative and long-term survival rates of c-VATS. Finally, because this study was initiated in 2006, during which both the 6th and the 7th editions of the TNM staging system were adopted, and although the 7th edition of the system was used during patient enrollment, the criteria used for assessing the efficacy of neoadjuvant therapy were not fully consistent. Thus, multi-center, prospective, randomized and controlled trials with larger sample sizes are warranted to clarify further the role of c-VATS following neoadjuvant therapy in the treatment of NSCLC.
In conclusion, c-VATS following neoadjuvant therapy is safe and feasible for the treatment of locally advanced NSCLC, and its long-term efficacy is satisfactory. In our current study, the application of multiple preoperative assessment methodologies, including fibrobronchoscopy, EBUS, PET-CT, mediastinoscopy, and chest CT, improved the outcomes by displaying the scope of the intrabronchial and peripheral tumor invasion and enabling the assessment of the effectiveness of the surgical regimens. Furthermore, the routinely performed intraoperative frozen pathological examination of the resection margins ensured a cancer-negative surgical margin and effectively reduced postoperative local recurrences.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [PubMed]
- Venuta F, Anile M, Diso D, et al. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg 2007;31:714-7. [PubMed]
- Steger V, Walker T, Mustafi M, et al. Surgery on unfavourable persistent N2/N3 non-small-cell lung cancer after trimodal therapy: do the results justify the risk? Interact Cardiovasc Thorac Surg 2012;15:948-53. [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Oparka J, Yan TD, Richards JM, et al. Video-assisted thoracoscopic pneumonectomy: The Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:105-8. [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. [PubMed]
- He J, Xu X. Thoracoscopic anatomic pulmonary resection. J Thorac Dis 2012;4:520-47. [PubMed]
- Liu J, Cai C, Wang D, et al. Video-assisted thoracoscopic surgery (VATS) for patients with solitary fibrous tumors of the pleura. J Thorac Oncol 2010;5:240-3. [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]
- He J, Xu X, Chen M, et al. Novel method to repair tracheal defect by pectoralis major myocutaneous flap. Ann Thorac Surg 2009;88:288-91. [PubMed]
- Giroux DJ, Rami-Porta R, Chansky K, et al. The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol 2009;4:679-83. [PubMed]
- Bepler G, Sommers KE, Cantor A, et al. Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer. J Thorac Oncol 2008;3:1112-8. [PubMed]
- Zheng Z, Chen T, Li X, et al. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med 2007;356:800-8. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Fujita S, Katakami N, Takahashi Y, et al. Postoperative complications after induction chemoradiotherapy in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2006;29:896-901. [PubMed]
- Tomaszek SC, Cassivi SD, Shen KR, et al. Clinical outcomes of video-assisted thoracoscopic lobectomy. Mayo Clin Proc 2009;84:509-13. [PubMed]
- Borro JM, Gonzalez D, Paradela M, et al. The two-incision approach for video-assisted thoracoscopic lobectomy: an initial experience. Eur J Cardiothorac Surg 2011;39:120-6. [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest 2005;128:2696-701. [PubMed]
- Witte B, Hürtgen M. Video-assisted mediastinoscopic lymphadenectomy (VAMLA). J Thorac Oncol 2007;2:367-9. [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]


