Early pleural fluid dynamics following video-assisted thoracoscopic lobectomy has limited clinical value
Introduction
The tolerated amount of pleural fluid produced prior to chest drain removal after thoracic surgery is an area of continued debate (1-4). Chest drains can even be avoided in selected cases after minor and major pulmonary surgery (5,6). With the introduction of the Thopaz+™ (Medela AG, Switzerland), new technology provides the clinician with detailed digital measurements of fluid production, in addition to the existing feature of air leak measurements. The change from traditional chest drainage devices to electronic devices has improved chest drain management due to reduced inter-observer variability of air leak, decreased chest drain duration and shorter length of stay (LOS) (7-9). Fluid production has previously been monitored using daily manual readings of fluid level, although shorter intervals have been recommended (10). Detailed measurements of fluid production in the Thopaz+™ may accommodate this need, and possibly improve drain management (11).
We hypothesized that digital assessment of pleural fluid dynamics in the early post-operative phase following VATS lobectomy could predict later fluid output.
Materials and methods
Patient selection
We examined detailed fluid curves from 50 patients undergoing VATS lobectomy for confirmed or suspected lung cancer to investigate whether fluid dynamics could predict fluid production at 24 or 48 hours after surgery. A 24-hour fluid production 4).
Surgical and post-operative management
All patients underwent VATS lobectomy under general anaesthesia using a standardized 3-port anterior approach (12). A 28-Fr chest drain was placed through the anterior port hole at the end of the procedure, and connected to an electronic drain box (Thopaz+™) with standard suction of −10 cmH2O. Decision on chest drain removal was done at the earliest on the morning round on post-operative day (POD) 1. Removal criteria were: Air leak consistently below 20 mL/min for 12 hours and a non-chylous, non-bloody fluid production <500 mL/24 hours. If air leak persisted above 4–5 days, suction was decreased at the discretion of the attending surgeon. Expanding subcutaneous emphysema was treated with elevated suction. Routine chest x-rays were performed after removal of the chest drain.
Data recorded
Baseline data were age, gender, body mass index (BMI), smoking status, lung function, lobe resected, duration of surgery, duration of chest drainage, LOS, pathology answer and cancer stage. Data on fluid production were extracted from the drain boxes using ThopEasy+©-software (Medela AG, Switzerland) and exported as a Microsoft Office Excel file. Some fluid values were higher than the actual volume in the reservoir due to a fluid film temporarily covering the sensor, causing spikes on the cumulative fluid curve. The actual fluid measured, after disappearance of the film, was used to eliminate these spikes. An average fluid production at 3-hour intervals was successively calculated.
Statistical analysis
Data were analysed and plotted using SAS software (Version 9.4, SAS Institute Inc., NC, USA). Prediction of fluid production ≥500 mL was calculated using Fisher’s exact test. Multiple logistic regression analysis was used to determine predictors of high 24-hour fluid output ≥500 mL during the first 24 hours of drainage. Continuous values are expressed as mean (SD) or median (IQR). Categorical values are expressed as number (%). A significance level of 0.05 was applied.
Compliance with ethical standards
The study was approved by the Danish Data Protection Agency (RH-2015-195, I-Suite no: 04126). All patients gave their consent to data collection. Due to the observational, non-interventional nature of the study, an approval by the National Review Board was not necessary.
Results
Between February 1 and June 24 2015, data on fluid production from 50 patients undergoing VATS lobectomy were retrieved. A total of 26 (52%) patients were classified as ‘low 24-hour fluid output’ defined as <500 mL/24 hours. The remaining 24 (48%) were classified as ‘high 24-hour fluid output’ defined as ≥500 mL/24 hours. Median drainage duration was 2.0 (1.0, 5.8) days for the whole group, and 1.1 in the ‘low’ and 4.9 in the ‘high 24-hour output’-group. Median LOS was 4.5 (2.0, 8.8) days for the whole group, and 3.0 in the ‘low’ and 6.5 in the ‘high 24-hour output’-group. Table 1 summarizes baseline data.
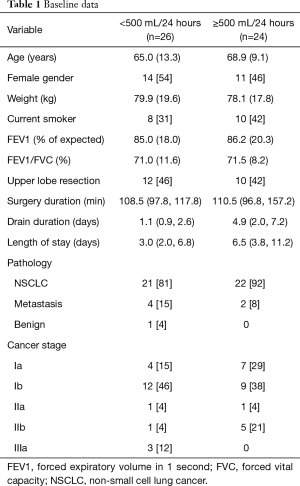
Full table
Figure 1 presents the cumulative fluid output of all patients until drain removal and Figure 2 the cumulative fluid output during the first 24 hours. Nine patients undergoing afternoon surgery had the drain removed after morning rounds on POD 1 between 20–23 hours after surgery. On visual assessment of the figures, the ‘low 24-hour fluid output’-group seemed to have a lower output within the first few hours compared with the ‘high 24-hour fluid output’-group. Consequently, a fluid production <200 mL during the first 6 post-operative hours was analysed for the predictive value of a ‘low 24-hour fluid output’, for the patients with a 24-hour measurement. Our results showed a positive predictive value (PPV) of 0.67 [95% confidence interval (CI): 0.46–0.83], due to 33% of patients with a 6-hour fluid production <200 mL ended with a ‘high 24-hour fluid output’. We found a statistically significant difference between groups <200 mL/6 hours and ≥200 mL/6 hours, and their respective ‘low’ and ‘high 24-hour fluid output’-groups (P<0.0001). Among the patients who had a fluid production ≥200 mL 6 hours after surgery, all 14 patients ended up with a ‘high 24-hour fluid output’. The calculated sensitivity of fluid measurements <200 and ≥200 mL/6 hours with regard to predicting 24-hour fluid output was 1 (95% CI: 0.78–1), but the specificity was 0.61 (95% CI: 0.39–0.80).
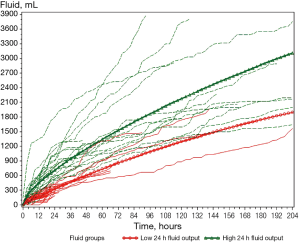
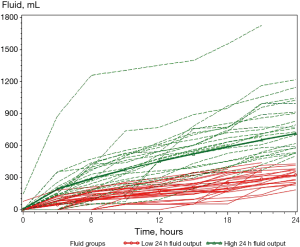
Fluid production at 24 hours was then analysed for predictive properties with regard to fluid output between 24 and 48 hours after surgery. Among the 9 patients with a ‘low 24-hour output’, 8 patients had a fluid production <500 mL in the period 24–48 hours after surgery giving a PPV of 0.89 (95% CI: 0.51–0.99). The NPV was 0.36 (95% CI: 0.14–0.64) and we found no statistically significant differences between groups when comparing ‘low’ and ‘high 24-hour fluid output’ with fluid output <500 and ≥500 mL 24–48 hours after surgery (P=0.3401).
Using multiple logistic regression, data on patients who had a drain for at least 24 hours after surgery, odds ratio and 95% confidence limits was calculated for gender, age, weight, upper lobe and surgery duration, and showed no significant prediction of fluid production during the first 24 hours after surgery. The odds ratio of surgery duration on fluid output was 1.032 (95% CI: 0.999–1.065, P=0.0588).
Discussion
In this study of 50 patients undergoing VATS lobectomy, our results showed that it was possible to predict a ‘high 24-hour fluid output’ using data 6 hours after surgery, although this does not seem to have a clinical impact on drain removal. The more clinically relevant ‘low 24-hour fluid output’ was not possible to predict using 6-hour post-operative fluid measurements (PPV=0.67). Similarly, data on 24-hour fluid output could not be used to predict later fluid output 24–48 hours after surgery. On logistic regression analysis of patient characteristics, we similarly did not find predictive values, and a possible trend in duration of surgery may be explained by an increased inflammatory response from longer procedures.
Although some surgeons have promoted conservative thresholds of fluid output before drain removal, accepted fluid criteria seem to have increased over time to 450–500 mL (1,2,4). Nevertheless, a recent randomized controlled trial favours a conservative fluid output below 300 mL per 24 hours (13). Possibly the divergence illustrates our lack in basic understanding of pleural fluid turnover in the complex pathophysiology of post-operative patients.
New medical devices have provided clinicians with valuable diagnostic and therapeutic tools throughout the history of medicine. Going from manual readings of pleural fluid at 24-hour intervals in the traditional water seal drain boxes to digital readings every hour with the newly developed Thopaz+™, the surgeon is provided with additional post-operative information of the patients. Furthermore, loss of useful data for ward rounds is prevented by the ability to graphically display the output history. In spite of these obvious benefits from a user point-of-view, a clinical benefit from precise fluid measurements, similar to the benefits of digital air leak measurements, has so far not been found.
This study is limited by the observational design and the sample size. Furthermore, specific levels of fluid production within the early post-operative phase were specified after the initial data collection, rendering the results to be ‘hypothesis-generating’ rather than conclusive. However, the strengths are the unprecedented detailed measurements and analyses with disappointing but relevant results.
Conclusions
Assessment of initial fluid production may predict high 24-hour fluid output, but continuous digital measurements of pleural fluid dynamics may have limited value in predicting fluid production after pulmonary lobectomy.
Acknowledgements
The authors wish to thank Morten Aagaard Petersen for statistical calculations and graphic presentations, and Kirstine Hartmann Johansen for data collection. This work was supported by Medela AG, Switzerland by providing electronic drainage devices during the study period. Bo Laksáfoss Holbek furthermore received a research grant from Medela for another study during the study period.
Footnote
Conflicts of Interest: Henrik Jessen Hansen reports personal fees from Medtronic, Medela, and Bard outside the submitted work. René Horsleben Petersen reports personal fees from Medtronic and Medela outside the submitted work. Professor Henrik Kehlet and Bo Laksáfoss Holbek have no conflict of interest.
References
- Younes RN, Gross JL, Aguiar S, et al. When to remove a chest tube? A randomized study with subsequent prospective consecutive validation. J Am Coll Surg. 2002;195:658-62. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg. 2008;135:269-73. [Crossref] [PubMed]
- Grodzki T. Prospective algorithm to remove chest tubes after pulmonary resection with high output--is it valid everywhere? J Thorac Cardiovasc Surg. 2008;136:536. [Crossref] [PubMed]
- Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 ml/day. Eur J Cardiothorac Surg. 2014;45:241-6. [Crossref] [PubMed]
- Holbek BL, Hansen HJ, Kehlet H, et al. Thoracoscopic pulmonary wedge resection without post-operative chest drain: an observational study. Gen Thorac Cardiovasc Surg. 2016;64:612-7. [Crossref] [PubMed]
- Ueda K, Hayashi M, Tanaka T, et al. Omitting chest tube drainage after thoracoscopic major lung resection. Eur J Cardiothorac Surg. 2013;44:225-9. [Crossref] [PubMed]
- Varela G, Jiménez MF, Novoa NM, et al. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg. 2009;35:28-31. [Crossref] [PubMed]
- Cerfolio RJ, Varela G, Brunelli A. Digital and smart chest drainage systems to monitor air leaks: the birth of a new era? Thorac Surg Clin. 2010;20:413-20. [Crossref] [PubMed]
- Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg. 2014;98:490-6; discussion 496-7. [Crossref] [PubMed]
- Brunelli A, Beretta E, Cassivi SD, et al. Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur. J. Cardiothorac. Surg. 2011;40:291-7. [Crossref] [PubMed]
- Holbek BL, Horsleben Petersen R, Kehlet H, et al. Fast-track video-assisted thoracoscopic surgery: future challenges. Scand Cardiovasc J. 2016;50:78-82. [Crossref] [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc. 2011;25:1263-9. [Crossref] [PubMed]
- Xie HY, Xu K, Tang JX, et al. A prospective randomized, controlled trial deems a drainage of 300 ml/day safe before removal of the last chest drain after video-assisted thoracoscopic surgery lobectomy. Interact Cardiovasc Thorac Surg. 2015;21:200-5. [Crossref] [PubMed]


