Dynamic expression of transformating growth factor-β1 and caveolin-1 in the lung of Bleomycin-induced interstitial lung disease
Introduction
Interstitial lung disease (ILD) is a disease with high mortality for the reason of that lung transplantation is generally considered as the only ultimate therapy. It affects the pulmonary parenchyma and is classified together based on specific clinical, radiological, and histopathological features. The pathological changes of histopathological features mainly include a heterogeneous group of disorders. Pathological examination showed diffuse alveolar inflammation and a lot of interstitial cell hyperplasia at first, then the collagen was deposited and leading to respiratory failure and death (1). Its pathogenesis remains unknown. Research shows that many factors can cause ILD incidence, according to the statistics, ILD secondary to connective tissue disease accounted for 19%-34% of all patients with ILD (2). Although ILD often occurs as clinically isolated, it commonly arises under the situation of an underlying connective tissue disease (UCTD) (3). These diseases include systemic sclerosis (SSc), where ILD occurs in a majority of patients, and rheumatoid arthritis (RA), polymyositis/dermato myositis (PM/DM), Sjögren’s syndrome, systemic lupus erythematosus (SLE), undierentiated CTD (UCTD) and mixed CTD (MCTD) where ILD is a less frequent complication (4).
Bleomycin induced ILD animal model is the most commonly used animal model (5,6) for revealing the cytokines and signal pathway in the pathogenesis of ILD. transforming growth factor-β1 (TGF-β1) is considered to be the well-known profibrotic cytokine. It promotes the formation of tissue fibrosis factor in the aspect of strongest expression, increase in fibrosis diseases and promote fibrosis foci inflammatory cell, fibroblast settled and aggregation (7,8). On the contrary, the expression of caveolin-1 (Cav-1) was observed to decrease in fibrosis disease (9-11), the vivo in vitro experiments have demonstrated the effect of anti-fibrosis (11,12), which suggesting a novel therapeutic target for patients with pulmonary fibrosis (13). In contrast to the published observation, CTD-ILD has become the focus of attention of clinical research. However currently the pathogenesis is unclear and there is still lack of effective treatment measures which can seriously affect the prognosis of the disease.
For CTD-ILD, the underlying pathology is dominated by inflammation or fibrosis, or the combination with both distinct radiologic and histopathologic patterns. In our previous clinical work, we found that different CTD-ILD would response to drug treatment differently, so we designed this experiment to observe the different expression of the two ILD in different periods. This study dynamically observed the lung inflammation and qualitative changing process of fibrosis pathology through the establishment of bleomycin induced rat pulmonary interstitial fibrosis model. At the same time, the expression of TGF-β1 and Cav-1 in the lung tissue was detected, and the relationship between the two proteins expression of lung lesions in different period of ILD was explored to further discover the pathogenesis of them in the aspect of ILD, which can provide theoretical and experimental basis for the development of effective ILD clinical decision making in the future.
Methods
Experimental animals
This study was carried out in strict accordance with the recommendations from the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing University of Chinese Medicine. Seventy-five male Wistar rats (age: mean 50 days, range, 45–55 days; weight: mean 220 g, range, 200–250 g) were obtained from the Dalian Medical University (license number: SCXK2013-0003). Rats were housed five per cage with free access to food and water and were allowed to acclimatize to the environment for three days prior to the start of the experiment. Rats were kept in an air conditioned room at 22 °C, 50% humidity and with a controlled 12 h light/dark cycle. The Department of Science and Technology of Liaoning Province has approved the animal study and relative experiment procedures.
Reagents
Bleomycin hydrochloride was purchased from Nippon Kayaku, CO, Japan; anti-TGF-β1 was purchased from Beijing Bioscience; anti-β-actin, anti-Cav-1 and secondary antibodies were obtained from Zhongshan Jinqiao.
Experimental design
Rats were randomly divided into two groups: an experimental group (n=60) and a control group (n=15). Rats were injected with 10% chloral hydrate intraperitoneal (4 mL/kg), incision of skin and soft tissue of the neck under aseptic conditions, exposing the trachea, obliquely piercing the trachea with 1 mL syringe under direct vision (14). The rats of control group were injected with normal saline 0.2 mL and the rats of experimental group were injected with Bleomycin 2.5 mg/kg (in 0.9% 0.2 mL physiological saline solution) (15). All animals were rotated upright rapidly. The rats were normal feed after being sutured. The rats of experimental group were sacrificed at days 7, 14, 21 and 28 by bleed to death, and the rats of control group were sacrificed at day 28. The right lung was taken to cryopreservation in liquid nitrogen immediately after the execution, at −80 °C refrigerator, for the analysis of RNA and protein. Soaking rat left lung in 10% formalin solution which is used for pathology analysis.
General state of rats
The rats’ weight, appetite, activity, respiration, hair, nose, sneezing and other changes were also observed after modeling.
Histopathology
The 1 cm × 1 cm pieces of left lung tissue were submerged in 10% formalin for at least 24 h, embedded in paraffin, and cut into 4 µm sections which were stained with hematoxylin and eosin (H&E) or Masson’s trichrome staining protocol to detect collagen fibers (15).
Immunohistochemistry was performed as well. The four µm tissue sections were washed three times in 0.5% BSA (Sigma-Aldrich) and blocked for 1 h in 2% BSA. After three washings of the samples in 0.5% BSA, tissue sections were incubated with rabbit anti-mouse Cav-1 antibody at 4 °C overnight. Afterwards, the slides were incubated for 1 hour with goat anti-rabbit IgG (Sigma-Aldrich). Samples were washed five times in BSA and PBS. Hoechst dye (Sigma-Aldrich) was added for 30 s, and samples were washed in PBS and mounted.
Western blotting
Right lung tissue was cut into 20 mg slices at −80 °C. Slices were incubated with total protein extraction solution and PMSF for 30 min on ice and centrifuged at 12,000 g for 10 min. Protein in supernatants was denatured in isopycnic loading buffer for 5 min at 100 °C. The 20 µL sample (2 µL protein) was subjected to electrophoresis on a 12% SDS-PAGE gel (200 v and 50 mA) and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with TBST buffer containing 5% skimmed milk powder for 3 h. Subsequently, the membrane was washed three times in TBST buffer, incubated with rabbit anti-mouse polyclonal anti-Cav-1, anti-TGF-β1 or anti-β-actin for 24 h at 4 °C, washed and incubated with HRP-conjugated goat anti-rabbit secondary antibody for 2 h at 24 °C. After being washed, immunoreactivity protein bands were visualized by using enhanced chemiluminescence and analyzed with the Gel-Pro Analyzer. Expression levels of Cav-1 and TGF-β1 of lung tissue were normalized to those of β-actin.
Real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells by using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized by using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgene Biotech, Beijing, China). In order to quantitate the expression of Cav-1 mRNA and TGF-β1 mRNA, qRT-PCR was performed by using TransStart Top Green qPCRSuperMix (Transgene Biotech). Oligonucleotide primers used for Cav-1 TGF-β1 and β-actin (internal control) were listed as following: Cav-1, 5'-TCC TGCTCT CCCGTTCCTT-3'(sense) and 5'-CGCCTCCCAGTCTTCCTATTT-3'(antisense); TGF-β1, 5'-TGAACCAAGGAGACGGAATACA-3'(sense) and 5'- GGAGCTGTGC AGGTGTTGAG-3'(antisense); β-actin, 5'-GGAGATTACTGCCCTGGCTCCT A-3'(sense) and 5'-GACTCATCGTACTCCTGCTTGCTG-3'(antisense). All samples were run in triplicate for target and internal control genes. Cycle threshold (Ct) values of Cav-1 cDNA and TGF-β1cDNA were normalized to β-actin by using the ΔΔCt method (16).
Statistical analysis
SPSS Statistics v.19 was used for statistical analyses. Data are expressed as means ± SD. Comparisons between groups were evaluated by using the independent t-test and the conditions of values <0.05 were considered as statistically significant.
Result
The general state of rats
After modeling, the rats in the experimental group appeared as decreased appetite, weight loss, messy and dim color hair, shortness of breath, around a week after beginning, the rats in the model group began to scratch the nose and sneezed.
The pathological changes
After modeling, in the experimental group, histopathological examination (HE) staining indicated that in alveolar septum, there were a large number of inflammatory cell infiltrations and alveolar structure destruction of normal form, accompanied by pulmonary bullae, Masson staining indicated that there was no obvious collagen deposition at day 7 compared to control group.
From day 14 to day 28, in the experimental group, HE staining indicated that the large alveolar structure was destroyed, the structure disappeared and the interstitial cells proliferated infiltration, while inflammatory cells gradually decreased and fibroblasts gradually increased. Masson staining indicated a large number of collagen fibers deposition (Figure 1), morphological quantitative analysis on the deposition of collagen fibre, displaying that the collagen deposition of day 14 was significantly increased than that of day 7, the peak appeared at day 21. Compared with the day 21, the collagen deposition of day 28 had no significant change (Figure 2).
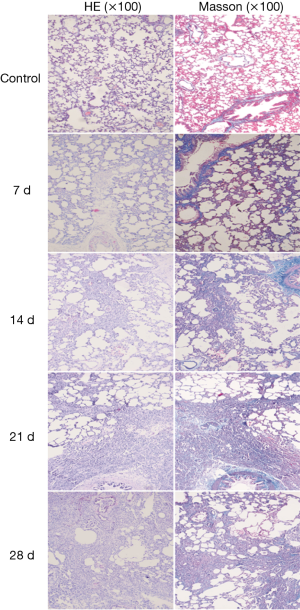
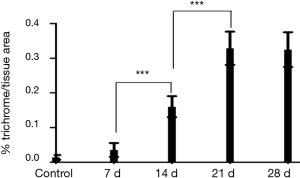
 , collagen fibers were stained area and lesion area ratio; ***, present P≤0.001.
, collagen fibers were stained area and lesion area ratio; ***, present P≤0.001.Immunohistochemistry
The expression of Cav-1 was observed in normal lung tissues especially in bronchial epithelial cells. After modeling, the expression of Cav-1 was decreased as time goes on after modeling (Figure 3).
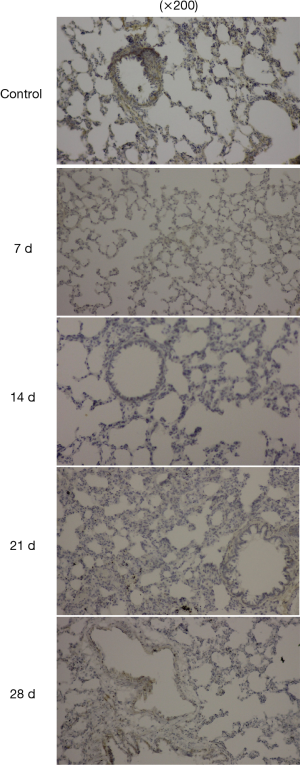
Western blot
The expression of TGF-β1 and Cav-1 protein was observed in normal lung tissues. After modeling, the TGF-β1 expression increased, the Cav-1 expression decreased, and at day 7, the expression of TGF-β1 and Cav-1 in the lung significantly changed (P<0.001). The expression of TGF-β1 at day 14 to day 28 maintained the highest level and the expression of Cav-1 reached the lowest level at 28 d (Figure 4).
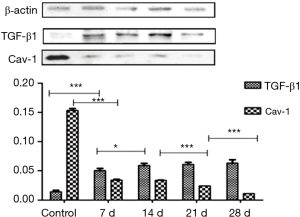
 . *, present P≤0.05; ***, present P≤0.001. TGF-β1, transforming growth factor-β1; Cav-1, caveolin-1.
. *, present P≤0.05; ***, present P≤0.001. TGF-β1, transforming growth factor-β1; Cav-1, caveolin-1.Real-time RT-PCR
With the development of inflammation and fibrosis, the expression of TGF-β1 mRNA at day 7 significantly increased in relative to normal lung tissue (P<0.001), peaked at day 21. At the same time, the expression level of Cav-1 mRNA declined significantly (P<0.001) at day 7, and reached the lowest level at day 21 (Figure 5).

 . *, present P≤0.05; ***, present P≤0.001. TGF-β1, transforming growth factor-β1; RT-PCR, real-time polymerase chain reaction; Cav-1, caveolin-1.
. *, present P≤0.05; ***, present P≤0.001. TGF-β1, transforming growth factor-β1; RT-PCR, real-time polymerase chain reaction; Cav-1, caveolin-1.Discussion
ILD is a challenging clinical entity associated with multiple CTDs and a significant cause of morbidity and mortality. Effective therapies for connective tissue disease-associated interstitial lung disease (CTD-ILD) are still lacking. There are six patterns of ILD: nonspecific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), desquamative interstitial pneumonia (DIP), cryptogenic organizing pneumonia (COP), dilluse alveolar damage (DAD), acute interstitial pneumonia (AIP) and lymphocytic interstitial pneumonia (LIP) (4). They have different characteristic radiographic findings on high resolution computerized tomography (HRCT) and histology. The immunosuppressive agents that most widely used for this purpose are corticosteroids, cyclophosphamide, azathioprine and mycophenolate mofetil. While corticosteroids are generally ineffective in UIP and other agents have demonstrated a modest beneficial effect (17).
The occurrence and development of lung lesion can be generally divided into three stages: damage, inflammation and repair (18). Intratracheal injection of bleomycin for establishing pulmonary interstitial lesion model of rats was induced by alveolar epithelial DNA damage, resulting in the release of a large number of inflammatory cells and cytokines, connecting paths between inflammation and fibrosis (19). In short term, the model is a good choice for the study of lung tissue injury, inflammation and fibrosis. Inflammation in the lung is the most common disease of pulmonary fibrosis promoting factors originating (19,20). Therefore, intervention and treatment in the early stage of inflammation may control the development of ILD. Chaudhary et al. (13) established bleomycin pulmonary interstitial fibrosis rat model, which instituted the anti-inflammatory intervention. The results showed that the given prednisone inhibited the inflammation and attenuated the development of fibrosis progression in inflammatory stage. But in the stage of fibrosis, inflammatory factor levels recovered to the normal level, the application of prednisone on the expression of collagen type I appeared no significant effect. So it is of great significance for the identification and treatment of ILD in early stage of inflammation.
In this study, we established bleomycin induced pulmonary interstitial lesion rat model. Inflammation and fibrosis of lung tissue in the experiment group were observed by HE and Masson staining. The results indicated that after modeling there were little inflammatory cell infiltration and collagen deposition in lung tissue at day 7. From the day 14 to day 28, the alveolar structure was damaged and lost, interstitial cell proliferation, aggregation as a focal distribution. HE staining indicated that inflammatory cells decreased gradually and the fibroblast increased gradually. Corresponding to Masson staining, collagen deposition increased by morphological quantitative analysis showed the deposition collagen fibers reached the peak at day 21. Mouratis et al. (6) reported that intratracheal injection of bleomycin induced pulmonary fibrosis rat model usually induces apoptosis of inflammatory reaction and epithelial cell death in the first week, and during the next 3 days which is the transition period, inflammation subsided gradually, in the meantime, the fibrosis was observed and the results showed that the fibrosis stage lasted until the 3–4 weeks. We established a model of pathological findings consistent with Mouratis et al. The represented study was to observe the time of inflammation and fibrosis, and to provide evidence for the time of drug intervention in the future. At the same time, the research demonstrated that the collagen deposition mainly occurred in the initial part of the inflammation, indicating that the formation and development in the model of inflammation might promote the fibrosis.
Caveolae are fask-shaped invaginated membrane vesicles with a usually diameter of 50–100 nm. There are three subtypes of it. Cav-1 is the most important member of the Caveolae family. It has many biological functions such as regulating intracellular signaling pathway, transduction and trans cell transport. The most abundant cav-1 expressing cells are fibroblasts, endothelial cells, type I pneumocytes, and adipocytes (21). Recent studies have shown that Cav-1 plays an important role in the pathogenesis of pulmonary interstitial diseases. Odajima et al. found that in mice with bleomycin induced pulmonary fibrosis and in patients with interstitial pneumonia, the expression of Cav-1 in the bronchioles decreased. Similarly, in patients with systemic sclerosis, the expression of Cav-1 in the skin and lung tissue decreased significantly; the lung and skin tissues of Cav-1 knockout mice became more fibrotic (22). Dynamic expression in the lung tissue by Western blot and real-time RT-PCR method for detection of TGF-β1 and Cav-1 indicated that TGF-β1 expression was up-regulated and down-regulated Cav-1 expression appeared at day 7 (inflammation); during the 21 to 28 d (collagen fibers deposition peak), the level of TGF-β1 reached the highest and the level of Cav-1 was the lowest. It indicates that TGF-β1 expression gradually increased and Cav-1 expression decreased might be closely related to the occurrence and development of ILD. Our results were consistent with the conclusion that the TGF-β1 and Cav-1 decreased in the previous studies of fibrosis. In addition, the changes in the expression of dynamic detection of TGF-β1, Chaudhary et al. showed which were the most significant pulmonary inflammation and fibrosis stage and TGF-β1 expression appeared a peak expression, also suggested that TGF-β1 is involved in the regulation of inflammation and fibrosis.
TGF-β1, as a multifunctional cytokine, regulating immune response in the early stage by increasing the expression of adhesion molecules and promoting inflammatory chemokines to recruit inflammatory cells, play a proinflammatory effect. Then it prevents the further development of inflammation, inducing fibroblast proliferation and extracellular matrix production by regulating T, B lymphocyte proliferation, differentiation and apoptosis (23,24), which plays an important role in the injury and repair of lung tissue (25). TGF-β1 regulate cell physiological and pathological reaction by multiple signaling pathways, mainly through the Smad signal transduction pathway activation of Smad 2, Smad 3 and Smad 4, and increasing expression of extracellular matrix protein gene by combining into the nucleus. Smad 6, 7 have negative feedback regulation on the path (26). In addition, TGF-β1 also involved in the occurrence and development of fibrosis through the MAPKs, NF-JB, K/Akt PI3 and other pathways. However, Cav-1 not only can down-regulate the expression of collagen protein and increase the expression of matrix metalloproteinase gene, but also can regulate the formation of fibrosis by linking to TGF-β1 signaling pathway. Mainly in the following four points: (I) the scaffolding domain in the molecular structure of Cav-1 combined with TGF-β1 receptor inhibite phosphorylation of downstream Smad signal; (II) Cav-1 can regulate expression of R I / II gene of TGF-β1; (III) Cav-1 can promote the internalization of TGF-β1 receptor; (IV) Cav-1 can activate TGF-β1 by modulating matrix metalloproteinases and integrin (27,28). Thus, Cav-1 plays a key role in the regulation of TGF-β1 induced fibrosis. In different fibrosis diseases, the expression of Cav-1 is decreased, while the expression of TGF-β1 is enhanced, which might provide a new idea for delaying the progress of fibrosis. But the TGF-β1 on Cav-1 regulatory mechanism is not fully understood yet (29-31).
In this experiment, tissue staining was used to observe the structure of the lung damage. If through electron microscope observation, the evidence of tissue injury can be observed more directly, and then we could provide a more objective basis by classifying and counting the inflammatory cells through the flow cytometry. In addition, we have not yet gone deeper on the TGF-β1 and Cav-1 signaling pathway exploration; therefore, the determination of the signaling pathway between them will provide evidence for the pathogenesis of ILD and relative drug intervention trials.
The ILD animal model induced by bleomycin is widely used in ILD research. Rats and mice are the most commonly used animals. Rats may have histopathology that is more reminiscent of IPF, although direct comparisons between rats and mice suggest similar responses to lung injury (32). But through this experiment, both inflammation period and fibrosis period were observed. In addition, the expressions of TGF-β1 and Cav-1 in different pathological stages were observed differently in this experiment. The expression of cytokines and Cav-1 varies greatly with different pathological stages. The bleomycin-induced rat model can be used as an ideal model to study novel target drugs of ILD. Hence, whether the interventions in different stages of the model can produce different outcomes or not will have a profound impact on the further study for the treatment of different ILD types especially for CTD-ILD.
CTD-ILD’s immune disorder causes persistent immune damage, as the repair process continues, it breaks the balance between extracellular matrix deposition and degradation. The balance between the two important regulatory factors, TGF-β1 and Cav-1, cannot be reconstructed, the regulatory effect of Cav-1 is suppressed and the fibrosis will continue. Due to the high morbidity and mortality of CTD-ILD, it is necessary to explore the pathogenesis of the disease and continue the development of new drugs. Increased expression of TGF-β1 and decreased expression of Cav-1 play a critical role in regulating the formation of fibrosis. Regulation of the two proteins is expected to be a new target for the future study, and also a challenge to find a safe and effective treatment method.
Acknowledgements
Funding: This study was supported by Natural foundation of Liaoning Province (2015020298).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by The Second Affiliated Hospital of Dalian Medical University ethics committee (No.201533).
References
- American Thoracic Society. European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest 2011;140:1292-9. [Crossref] [PubMed]
- Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet 2012;380:689-98. [Crossref] [PubMed]
- Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther 2010;12:213. [Crossref] [PubMed]
- Moeller A, Ask K, Warburton D, et al. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 2008;40:362-82. [Crossref] [PubMed]
- Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med 2011;17:355-61. [Crossref] [PubMed]
- Gauldie J, Jordana M, Cox G. Cytokines and pulmonary fibrosis. Thorax 1993;48:931-5. [Crossref] [PubMed]
- Edwards DR, Murphy G, Reynolds JJ, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J 1987;6:1899-904. [PubMed]
- Del Galdo F, Sotgia F, de Almeida CJ, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum 2008;58:2854-65. [Crossref] [PubMed]
- Wang XM, Zhang Y, Kim HP, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 2006;203:2895-906. [Crossref] [PubMed]
- Tourkina E, Richard M, Gööz P, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 2008;294:L843-861. [Crossref] [PubMed]
- Gvaramia D, Blaauboer ME, Hanemaaijer R, et al. Role of caveolin-1 in fibrotic diseases Matrix Biology 2013;32:307-15. [Crossref] [PubMed]
- Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 2006;173:769-76. [Crossref] [PubMed]
- B Moore B, Lawson WE, Oury TD, et al. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 2013;49:167-79. [Crossref] [PubMed]
- Akgedik R, Akgedik S, Karamanl H, et al. Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 2012;35:1732-41. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Lynch JP, Saggar R, Weigt SS, et al. Usual interstitial pneumonia. Semin Respir Crit Care Med 2006;27:634-51. [Crossref] [PubMed]
- Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2009;2:103-21. [Crossref] [PubMed]
- Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol 1991;65:81-94. [Crossref] [PubMed]
- Izbicki G, Segel MJ, Christensen TG, et al. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol 2002;83:111-9. [Crossref] [PubMed]
- Jin Y, Lee SJ, Minshall RD, et al. Caveolin-1: a critical regulator of lung injury. Am J Physiol Lung Cell Mol Physiol 2011;300:L151-60. [Crossref] [PubMed]
- Odajima N, Betsuyaku T, Nasuhara Y, et al. Loss of caveolin-1 inbronchiolization in lung fibrosis. J Histochem Cytochem 2007;55:899-909. [Crossref] [PubMed]
- Aoki CA, Borchers AT, Li M, et al. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun Rev 2005;4:450-9. [Crossref] [PubMed]
- Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 2015;24:102-14. [Crossref] [PubMed]
- Luo F, Zhuang Y, Sides MD, et al. Arsenic trioxide inhibits transforming growth factor-β1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir Res 2014;15:51. [Crossref] [PubMed]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685-700. [Crossref] [PubMed]
- Shi Y, Gochuico BR, Yu G, et al. Syndecan-2 exerts antifibrotic effects by promoting caveolin-1-mediated transforming growth factor-β receptor I internalization and inhibiting transforming growth factor-β1 signaling. Am J Respir Crit Care Med 2013;188:831-41. [Crossref] [PubMed]
- Sanders YY, Cui Z, Le Saux CJ, et al. SMAD-independent down-regulation of caveolin-1 by TGF-β: effects on proliferation and survival of myofibroblasts. PLoS One 2015;10:e0116995. [Crossref] [PubMed]
- Gvaramia D, Blaauboer ME, Hanemaaijer R, et al. Role of caveolin-1 in fibrotic diseases. Matrix Biol 2013;32:307-15. [Crossref] [PubMed]
- Miyasato SK, Loeffler J, Shohet R, et al. Caveolin-1 modulates TGF-β1 signaling in cardiac remodeling. Matrix Biol 2011;30:318-29. [Crossref] [PubMed]
- Lu GX, Bian DF, Ji Y, et al. Madecassoside ameliorates bleomycin-induced pulmonary fibrosis in mice by downregulating collagen deposition. Phytother Res 2014;28:1224-31. [Crossref] [PubMed]
- Jenkins RG, et al. An Official American Thoracic Society Workshop Report: Use of Animal Models for the Preclinical Assessment of Potential Therapies for Pulmonary Fibrosis. Am J Respir Cell Mol Biol 2017;56:667-79. [Crossref] [PubMed]


