Management of pulmonary hypertension from left heart disease in candidates for orthotopic heart transplantation
Introduction
Pulmonary hypertension in left heart disease (PH-LHD) commonly complicates prolonged heart failure (HF). Patients undergoing orthotopic heart transplant (OHT) with PH-LHD, and specifically out of proportion PH, which is a combination of pre-and post-capillary PH, have increased morbidity and mortality. An elevated pulmonary vascular resistance (PVR) >2.5 Wood units results in a nearly 30% increase in mortality within the first month post-transplant (1,2). In this review, we examine the data published between January, 1990 to present day regarding the definition and prognosis of this disease as well as both medical and mechanical support therapeutic strategies. The only guideline recommended treatment of PH-LHD is aimed at treating the underlying HF by reducing the pulmonary capillary wedge pressure (PCWP) and potentially allowing the normalization of PVR over time. These treatments for HF are well established and include diuretics, angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists, ARB/neprilysin inhibitor, beta-blockers, and in certain populations, non-specific vasodilators such as nitrates and hydralazine. In patients with low cardiac output, inotropic drugs may be required. For patients with longstanding HF, mechanical support with left ventricular assist devices (LVAD) has demonstrated a benefit by unloading the left ventricle (LV) and normalizing PVR, especially in candidates for OHT (3,4).
Some medications used for the treatment of World Health Organization (WHO) group 1 pulmonary arterial hypertension (WHO PAH classification, Table 1) have been employed off-label in the setting of WHO group 2 PH-LHD, perhaps driven by the hypothesis that there is a significant PAH-like component in out of proportion PH. These studies have demonstrated variable efficacy and often negative outcomes in a strategy to optimize the PVR pre-OHT. Specifically, the use of guanylyl cyclase pathway modulators, including phosphodiesterase 5 (PDE-5) inhibitors appear to be more successful than others, especially in the peri-operative period.
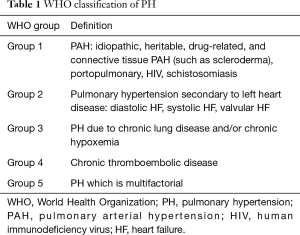
Full table
Definition of pulmonary hypertension
PH is a frequent comorbidity in patients with prolonged HF. Nearly two thirds of patients with HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF) have PH (5). Comorbid PH in patients with HFrEF increases risk of HF hospital admissions and in patients with HFpEF it is associated with an increase in 5-year all-cause mortality (6). In patients with advanced HF, the mechanism of PH is related to a post-capillary and passive rise in mean pulmonary artery pressure (mPAP) resulting from a back-transmission of elevated LV diastolic pressure (7). This type of PH is defined invasively by mPAP >25 mmHg, and PCWP >15 mmHg, with a diastolic pressure gradient (DPG; defined as diastolic PA pressure—mean PCWP) <7 mmHg (3).
In patients with prolonged HF and WHO Group 2 PH, the subtype of combined pre-and post-capillary PH, as defined at mPAP >25 mmHg, PCWP >15 mmHg, and DPG >7, is frequently present. In addition to the passive rise in mPAP resulting from increased LV hydrostatic pressures (post-capillary PH), the pre-capillary component has characteristics of WHO group 1 PH, in which there is vascular remodeling due to multiple pathologic changes in biochemical signaling: a decrease in endogenous nitric oxide (NO), increased endothelin expression, and desensitization to natriuretic peptide induced vasodilatation (3). These pathophysiologic mechanisms result in elevation of mPAP in excess of PCWP, giving the names of “fixed”, “out of proportion”, “mixed” or “combined pre-and post-capillary” PH to this disease entity. Throughout this review we will employ the term “out of proportion” PH. Hemodynamically, this is characterized by a PVR >5 Wood units, transpulmonary gradient (TPG) [mPAP minus the PCWP] >15 mmHg, non-reversible hemodynamics by administration of pulmonary vasodilators such as nitroprusside (8,9). Further stratification of patients with mixed PH as those having increased or normal pulmonary DPG may have treatment and prognostic significance, with a DPG >7 mmHg indicating advanced pulmonary vascular remodeling and mixed pre-and post-capillary PH (3,10,11).
PH in advanced HF and clinical outcomes
The presence of out of proportion PH has clinical and prognostic significance for patients with longstanding HF being considered for OHT due to elevated PVR, chronic RV overload, and an increased risk of post-transplant right HF. This has negatively impacted survival in the first year post transplant (1). Current International Society for Heart and Lung Transplantation (ISHLT) guidelines consider PH that is not readily reversible by pulmonary vasodilators, or “fixed PH”, to be a relative contraindication to OHT (12-14). A review of the Cardiac Transplant Research Database (CTRD) consisting of patient data from 26 US institutions from the past 20 years reveals that the overall risk of death after OHT has decreased and, in particular, the contribution of elevated PVR to increased mortality has declined over time from over 10% to less than 5% at one year post transplant (15). Similar trends in post-OHT outcomes are also observed from the Registry of ISHLT (16). A reason for this important trend could be the result of a combination of guideline directed treatment for Group 2 PH (4) as well as institution-specific use of off-label Group 1 PH therapies (17) in select patients with a normalization of PCWP on medical or mechanical circulatory assist therapy. We will focus on the studies to date that have examined the efficacy and outcomes of Group 1 PH therapies in patients with Group 2 PH. Where sufficient data is available, the discussion of treatment of Group 2 PH will emphasize three categories of patients: patients with HF (irrespective of presence of PH), patients with HF and PH, and patient with HF and PH undergoing optimization for OHT.
Treatment of PH-LHD
Vasodilators—inhaled and intravenous
Pulmonary selective vasodilators have been used for several decades in the treatment of longstanding PH-LHD. Their use in the acute setting has been validated by basic and clinical science, but with no randomized controlled studies showing a definitive benefit (18,19). These agents act as selective pulmonary vasodilators by increasing cyclic guanosine monophosphate resulting in an improvement of ventilation-perfusion matching and a reduction in PVR, although as a class there may be a less pronounced effect on lowering TPG (17,20). Most commonly used are inhaled nitric oxide (NO) and inhaled prostacyclins (for example, iloprost). Both medications may be useful in the acute peri-operative setting in patients with HF and PH (21-23). Argenziano et al. [1998] randomized 11 of 23 consecutive patients post LVAD placement who met criteria for elevated PVR to either NO or inhaled nitrogen therapy. Significantly, only the 6 patients who received NO exhibited significant reductions in mean PA pressure from 35±6 to 24±4 mmHg while the 5 patients receiving inhaled nitrogen had no significant change in mean PA pressure (21). In a study comparing NO and inhaled prostacyclin in post-OHT and post lung transplant patients, both agents were found to similarly reduce mPAP, central venous pressure and improve cardiac index (CI) (24). In a head to head comparison of NO and inhaled iloprost, a prostaglandin, in 46 patients being weaned from cardiopulmonary bypass, both agents reduced PVR, mPAP and increased cardiac output, with the latter agent being significantly more effective (25). Iloprost has some advantages over NO as there is no risk of methemoglobinemia or rebound PH with prolonged use (17,20). NO requires continuous inhalation given its very short half-life, with even a brief interruption resulting in rebound PH and potential RV failure. Iloprost may be given at doses of 5–10 mcg every 3–4 h and can be weaned off in the perioperative period (26). While current published studies suggest a clinical benefit in the perioperative setting, there is no evidence to support chronic therapy with inhaled vasodilators in HF or HF with PH patients given the cost and difficulty of administration.
Intravenous agents such as nitroglycerin and nitroprusside, both exogenous NO donors, are commonly used in the acute setting to assess reversibility of PH-LHD and as a bridge therapy to more definitive treatments (17,27,28). In a study of 33 patients with PH secondary to end-stage HF, the use of nesiritide, a synthetic B-type natriuretic peptide, during hospitalization for HF significantly reduced PCWP by 31% and mPAP by 15.6% compared to pre-treatment (29). Michaels et al. [2005] studied 20 patients with HF and PH (mPA >25 mmHg): 10 with PCWP >15 mmHg and 10 with PCWP ≤15. In the patients with what the researchers defined as post-capillary PH or PH with a PCWP >15, nesiritide infusion for 30 minutes decreased both the mPA pressure and PCWP, and significantly increased the CI (30). However, the long term therapeutic benefit of nesiritide in pre-OHT patients is uncertain. The intravenous form of epoprostenol appears to have benefit in selected patients in the peri- or post-transplant period but not as long term therapy prior to transplant while the patient has LV dysfunction. Califf et al. [1997], in The Flolan International Randomized Survival Trial (FIRST), enrolled 471 patients with New York Heart Association (NYHA) class IIIB or IV HF. With the use of intravenous epoprostenol, hemodynamic factors such as mPAP, PCWP, and PVR improved acutely however the long-term infusion not only failed to demonstrate clinical benefits but also showed a trend toward increased mortality (31).
PDE-5 inhibitors and guanylyl cyclase agonists
The modulators of the guanylyl cyclase pathway in the endothelium have overall shown the greatest promise of treatment efficacy for PH-LHD. Redfield et al. [2013] studied the effect of sildenafil, a PDE-5 inhibitor on exercise capacity in 216 patients with HFpEF (EF >50%). The patients were randomized to receive either sildenafil or placebo for a total of 24 weeks. At 24 weeks peak oxygen consumption and change in 6-minute walk test were not significantly different between the two groups (32). Several randomized trials are completed or currently underway studying the treatment efficacy of PDE-5 inhibitors in the PH-LHD population (Table 2). Within the same general class of guanylyl cyclase modulator therapies, novel guanylyl cyclase stimulators are showing promise. Riociguat is a soluble guanylate cyclase stimulator which demonstrated positive hemodynamic effects in 2 randomized trials by Bonderman et al. (2013 and 2014) in patients with HF and secondary PH. Although neither trial met the primary endpoint of change in mPAP, riociguat treatment did result in an increase in CI, stroke volume and decrease in systemic and peripheral vascular resistance (Table 2) (37,38).
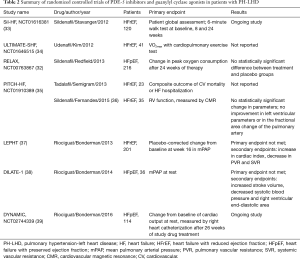
Full table
A study by Lewis et al. [2007] in patients with HF and PH demonstrated that the PDE-5 inhibitor sildenafil improved NYHA class, 6-minute walk distance, as well as significantly reduced hospitalizations for HF (40). In a retrospective study by de Groote et al. [2015] sildenafil use pre-OHT in 18 patients resulted in a change in PVR at an average of 8.7 months of treatment from 5.3±1.9 Wood units to 3.3±1.8 Wood units (P=0.01). Five patients were able to proceed with successful OHT and 6 with left ventricular assist device (LVAD). All patients with a PVR <3 Wood units after treatment survived, with survival in the group with PVR >3 being only 44% (41). In addition, hemodynamic parameters such as RV ejection fraction, PVR, and peak oxygen consumption improved in this population of HF patients with WHO group 2 PH with the administration of sildenafil (41). The effect of sildenafil to optimize the PVR in 119 consecutive patients was studied at one institution by Pons et al. [2012]. Fifteen patients with a PVR >2.5 Wood units and/or TPG >12 were treated with sildenafil pre-operatively with a target dose of 109±42 mg/day of sildenafil (titrated and administered as three times a day dosing) for 163±116 days prior to OHT. Hemodynamic parameters improved in these patients: mPAP, PVR, and TPG all decreased significantly in this group at risk for right HF post-OHT. Specifically, mPAP decreased from 43.9±12.5 to 33.4±5.8 mmHg and PVR decreased from 5±1.1 to 3±1.6 (P<0.01) Wood units in the 15 patients treated with sildenafil pre-operatively. Post-OHT mortality was comparable between the two groups of patients with and without severe PH-LHD in 6 month follow-up (42).
Endothelin receptor antagonists (ERAs)
Endothelin is a potent endogenous vasoconstrictor with both pulmonary and systemic effects. It is upregulated in patients with prolonged HF and correlates with more severe PH, increased PVR, and worse survival outcomes. Preclinical and small clinical studies of ERA have shown improvements in hemodynamic parameters and morbidity. Administration of the non-specific endothelin receptor A and B (ETA and ETB) antagonist, bosentan, in a study of more than 80 end-stage HF patients, increased by 20% the number of eligible patients for OHT by reducing mPAP, PVR and TPG, as compared to the control group. One-year survival on the transplant waiting list was also significantly improved in this study (43). Despite initial encouraging results, randomized trials of a non-selective ERA, tezosentan, studied in the Randomized Intravenous Tezosentan (RITZ) trial and the Value of Endothelin Receptor Inhibition with Tezosentan in Acute Heart Failure Study (VERITAS) trial have failed to show lasting positive effect on outcomes and clinical symptoms in patients with advanced HF (44-48). The Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure (ENABLE) trial studied 1,000 patients with NYHA IIIB and IV treated with low-dose bosentan did not show differences in the primary endpoint of all-cause mortality and HF hospitalization (49). In fact, one study reported a high incidence of liver abnormalities (50). Non-randomized studies of selective ETA receptor antagonists darusentan and sitaxsentan in patients with HFrEF have demonstrated improved hemodynamics without substantial side effects (51,52). However, in the Endothelin A Receptor Antagonist Trial in Heart Failure (EARTH) trial, darusentan did not provide a sustainable benefit on LV remodeling, morbidity or mortality in 642 patients with chronic HF (53). Due to the overall negative results from larger, randomized trials, the guidelines do not recommend use of the ERAs in patients with HF and PH (4,5,12). A randomized trial evaluating the safety and tolerability of macitentan, another non-specific ERA, in patients with Group 2 PH from HrEF is currently underway (54). Newer ERAs including ambrisentan and macitentan may have a better hepatic safety profile than bosentan and sitaxsentan, and it is unclear if there will be a role for these drugs in patients listed for OHT in the optimization of their PVR and TPG pre-transplant. One common class side-effect of ERAs is volume retention, which may complicate the treatment of HF. Additionally, it is unclear if these PAH specific medications would have a better safety profile and outcome if the patients were closely monitored with pulmonary artery catheters, ensuring their PCWP was <15 mmHg during the use of off-label PAH medication.
Phosphodiesterase 3 (PDE3) inhibitors
PDE3 inhibitors such as milrinone and enoximone cause direct vasodilation in the pulmonary and systemic circulation, predominantly increasing flow with smaller changes in TPG (17). Despite these nearly instantaneous hemodynamic effects, this class of medications has not been shown to improve clinical outcomes, and, in fact, increases mortality in HF patients. Two large randomized trials with a combined >1,000 patients [The Prospective Randomized Milrinone Survival Evaluation (PROMISE) and The Studies of Oral Enoximone Therapy in Advanced Heart Failure (ESSENTIAL)] did not show benefit of these agents in patients with NYHA class III-IV HF (55,56). In addition, neither of these studies included patients listed for OHT.
Combination of medical therapy and mechanical support
A number of patients with medically refractory PH-LHD have improved outcomes with the use of mechanical circulatory support. Seventy percent of this group are successfully bridged to transplant and their clinical outcomes and survival are comparable at 30 days and 12 months to patients without out of proportion PH (12,57,58). Hemodynamic improvement is seen early after implant with mPAP and PVR decreasing significantly within days (58-62). Kutty et al. [2013] demonstrated these hemodynamic changes in their study of 29 patients implanted with centrifugal LVADs. Baseline and post-VAD pulmonary hemodynamics were significantly improved. TPG was reduced from 14±3.9 to 9±3.3 mmHg and PVR decreased from 5±1.5 to 2.1±0.5 Wood units (P<0.05) post LVAD (58). These hemodynamic effects were also observed by John et al. [2010], in a cohort of 50 patients who received a continuous-flow HeartMate II LVAD. Mean PVR decreased significantly from a baseline of 3.6±1.9 to 2.1±0.8 Wood units (P<0.001) (59). Etz et al. [2007] demonstrated not only improvement in post-VAD pulmonary hemodynamics but in addition more than half of the patients were successfully bridged to OHT (63). Mikus et al. followed 145 patients, studying the hemodynamic effects of circulatory support from time of implant to beyond 1 year. 56 patients had out of proportion PH, with a baseline PVR of 3.49±1.47 Woods units, which after LVAD support was reduced to 1.4±0.7 and 1.7±0.6 Wood units, at 6 and 12 months, respectively (60). There are currently no randomized clinical trials looking at the combined use of WHO group 1 PH medical therapies and LVAD in patients with out of proportion PH being optimized for OHT. Recent data suggest a benefit with the concomitant use of PDE-5 inhibitors, in particular sildenafil. In the perioperative setting, using sildenafil improves pulmonary hemodynamics and significantly increases cardiac output (64). Post LVAD implantation, weaning vasodilators such as NO could be challenging due to the side-effects of rebound PH and decreased RV function. With the addition of sildenafil, all patients in the study by Klodell et al. [2007] were weaned off NO and inotropic therapies. A lasting reduction in PA systolic pressure was observed as early as 90 minutes after the administration of oral sildenafil (65). More than 40% of the patients in a study by Tedford et al. [2008] had reduced PVR <3–3.5 Wood units with the combination of LVAD and sildenafil therapy, allowing them to become OHT candidates (66).
There are less published data on the effectiveness of inhaled vasodilators and ERA post-LVAD and prior to OHT. A small study of 7 patients post-LVAD demonstrated that administering NO and iloprost significantly reduced PVR, mPAP, RV systolic pressure, PCWP, and improved the efficiency of the assist device by increasing LVAD flow within 2 h after the initiation of these medications. In all seven patients, RVAD was avoided (67). In another study of bosentan, 50 consecutive patients were treated post LVAD. About 20% had to discontinue the medication because of side effects and the rest of the patients completed the study and had significant improvement in hemodynamics (68). Further studies are under way to explore the use of ERAs in patients with pre-existing PH-LHD and subsequently normalized PCWP, as is the case in post-LVAD or OHT. One such study which is enrolling will examine the efficacy and safety of macitentan in patients with PH after VAD in patients with RV failure (69).
Conclusions
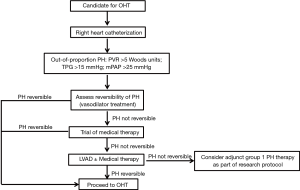
PH-LHD remains a significant risk factor for morbidity and mortality in patients being evaluated for OHT. A standardized classification and definition of the disease is still lacking, adding additional complexity for patient assessment and development of studies to assess treatment efficacy and safety. The sub-type of out of proportion PH-LHD is difficult to treat and is associated with the worst patient outcomes. Several medical therapies mostly used in the treatment of WHO Group 1 PH have been used off-label in the treatment of PH-LHD with varying success. To date, the most promising results in this category suggest that vasodilator therapies could be employed in the acute and perioperative settings and modulators of the guanylyl cyclase pathway could be used in more stable patients prior to OHT. Based on the available data we have presented, we propose a stepwise approach in the evaluation and management of patients with PH-LHD being evaluated for OHT (Figure 1). We hypothesize that there may be a role for group 1 PH therapies in the treatment of PH-LHD after normalization of PCWP whether by standard HF medical therapy or mechanical circulatory support or the concomitant use of both. However, given the available evidence at this time, this review cannot justify the off-label use of Group 1 PH medications such as PDE-5 inhibitors, guanylyl cyclase stimulators, ERAs, or prostacyclins prior to OHT in the treatment of PH-LHD, unless it is part of a research protocol. The strongest evidence of treatment of PH-LHD prior to OHT remains the use of standard medical HF therapy and LVAD support. Future research and randomized clinical trials are needed to increase our understanding of the potential role of group 1 PH therapies as an additional strategy to optimize patients with PH-LHD pre-OHT.
Acknowledgements
None.
Footnote
Conflicts of Interest: Drs. Koulova, Patibandla, Gupta, and Aronow have no conflicts of interest to declare. Dr. Gass is on the speaker’s bureau for Novartis and Zoll. Dr. Lanier is a member of the speakers’ bureau for Actelion, Bayer, Gilead, and United Therapeutics.
References
- Vakil K, Duval S, Sharma A, et al. Impact of pre-transplant pulmonary hypertension on survival after heart transplantation: A UNOS registry analysis. Int J Cardiol 2014;176:595-9. [Crossref] [PubMed]
- Murali S, Kormos RL, Uretsky BF, et al. Preoperative pulmonary hemodynamics and early mortality after orthotopic cardiac transplantation: the Pittsburgh experience. Am Heart J 1993;126:896-904. [Crossref] [PubMed]
- Vachiéry JL, Adir Y, Barberà JA, et al. Pulmonary Hypertension Due to Left Heart Diseases. J Am Coll Cardiol 2013;62:D100-8. [Crossref] [PubMed]
- Writing Committee Members, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-e327. [Crossref] [PubMed]
- "2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)." Nazzareno Galiè, Marc Humbert, Jean-Luc Vachiery, Simon Gibbs, Irene Lang, Adam Torbicki, Gérald Simonneau, Andrew Peacock, Anton Vonk Noordegraaf, Maurice Beghetti, Ardeschir Ghofrani, Miguel Angel Gomez Sanchez, Georg Hansmann, Walter Klepetko, Patrizio Lancellotti, Marco Matucci, Theresa McDonagh, Luc A. Pierard, Pedro T. Trindade, Maurizio Zompatori and Marius Hoeper. Eur Respir J 2015; 46: 903-975. Eur Respir J 2015;46:1855-6. [PubMed]
- Salamon JN, Kelesidis I, Msaouel P, et al. Outcomes in World Health Organization Group II Pulmonary Hypertension: Mortality and Readmission Trends With Systolic and Preserved Ejection Fraction–Induced Pulmonary Hypertension. J Card Fail 2014;20:467-75. [Crossref] [PubMed]
- Nagy AI, Venkateshvaran A, Merkely B, et al. Determinants and prognostic implications of the negative diastolic pulmonary pressure gradient in patients with pulmonary hypertension due to left heart disease. Eur J Heart Fail 2017;19:88-97. [Crossref] [PubMed]
- Guazzi M, Borlaug BA. Pulmonary Hypertension Due to Left Heart Disease. Circulation 2012;126:975-90. [Crossref] [PubMed]
- Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation 2010;122:173-83. [Crossref] [PubMed]
- Gerges C, Gerges M, Lang MB, et al. Diastolic Pulmonary Vascular Pressure Gradient. Chest 2013;143:758-66. [Crossref] [PubMed]
- Tampakakis E, Leary PJ, Selby VN, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail 2015;3:9-16. [Crossref] [PubMed]
- Mehra MR, Kobashigawa J, Starling R, et al. Listing Criteria for Heart Transplantation: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates—2006. J Heart Lung Transplant 2006;25:1024-42. [Crossref] [PubMed]
- Chen JM, Levin HR, Michler RE, et al. Reevaluating the significance of pulmonary hypertension before cardiac transplantation: determination of optimal thresholds and quantification of the effect of reversibility on perioperative mortality. J Thorac Cardiovasc Surg 1997;114:627-34. [Crossref] [PubMed]
- Lindelöw B, Andersson B, Waagstein F, et al. High and low pulmonary vascular resistance in heart transplant candidates. A 5-year follow-up after heart transplantation shows continuous reduction in resistance and no difference in complication rate. Eur Heart J 1999;20:148-56. [Crossref] [PubMed]
- Tallaj JA, Pamboukian SV, George JF, et al. Have risk factors for mortality after heart transplantation changed over time? Insights from 19 years of Cardiac Transplant Research Database study. J Heart Lung Transplant 2014;33:1304-11. [Crossref] [PubMed]
- The Registry of the International Society for Heart and Lung Transplantation. 2017. Available online: https://www.ishlt.org/registries/heartLungRegistry.asp
- Fang JC, DeMarco T, Givertz MM, et al. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult--a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012;31:913-33. [Crossref] [PubMed]
- Yin N, Kaestle S, Yin J, et al. Inhaled nitric oxide versus aerosolized iloprost for the treatment of pulmonary hypertension with left heart disease. Crit Care Med 2009;37:980-6. [Crossref] [PubMed]
- Matsumoto A, Momomura S, Sugiura S, et al. Effect of inhaled nitric oxide on gas exchange in patients with congestive heart failure. A randomized, controlled trial. Ann Intern Med 1999;130:40-4. [Crossref] [PubMed]
- King C, May CW, Williams J, et al. Management of Right Heart Failure in the Critically Ill. Crit Care Clin 2014;30:475-98. [Crossref] [PubMed]
- Argenziano M, Choudhri AF, Moazami N, et al. Randomized, Double-Blind Trial of Inhaled Nitric Oxide in LVAD Recipients With Pulmonary Hypertension. Ann Thorac Surg 1998;65:340-5. [Crossref] [PubMed]
- Ardehali A, Hughes K, Sadeghi A, et al. Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation 2001;72:638-41. [Crossref] [PubMed]
- Mahajan A, Shabanie A, Varshney SM, et al. Inhaled nitric oxide in the preoperative evaluation of pulmonary hypertension in heart transplant candidates. J Cardiothorac Vasc Anesth 2007;21:51-6. [Crossref] [PubMed]
- Khan TA, Schnickel G, Ross D, et al. A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg 2009;138:1417-24. [Crossref] [PubMed]
- Winterhalter M, Simon A, Fischer S, et al. Comparison of inhaled iloprost and nitric oxide in patients with pulmonary hypertension during weaning from cardiopulmonary bypass in cardiac surgery: a prospective randomized trial. J Cardiothorac Vasc Anesth 2008;22:406-13. [Crossref] [PubMed]
- Olschewski H, Simonneau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002;347:322-9. [Crossref] [PubMed]
- Lim HS, Zaphiriou A. Sodium Nitroprusside in Patients With Mixed Pulmonary Hypertension and Left Heart Disease: Hemodynamic Predictors of Response and Prognostic Implications. J Card Fail 2016;22:117-24. [Crossref] [PubMed]
- Pasero D, Rana NK, Bonato R, et al. Inhaled nitric oxide versus sodium nitroprusside for preoperative evaluation of pulmonary hypertension in heart transplant candidates. Transplant Proc 2013;45:2746-9. [Crossref] [PubMed]
- O'Dell KM, Kalus JS, Kucukarslan S, et al. Nesiritide for secondary pulmonary hypertension in patients with end-stage heart failure. Am J Health Syst Pharm 2005;62:606-9. [PubMed]
- Michaels AD, Chatterjee K, De Marco T. Effects of intravenous nesiritide on pulmonary vascular hemodynamics in pulmonary hypertension. J Card Fail 2005;11:425-31. [Crossref] [PubMed]
- Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am Heart J 1997;134:44-54. [Crossref] [PubMed]
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268-77. [Crossref] [PubMed]
- Sildenafil Versus Placebo in Chronic Heart Failure (SilHF). Available online: https://clinicaltrials.gov/ct2/show/NCT01616381
- Udenafil Therapy to Improve Symptomatology, Exercise Tolerance and Hemodynamics in Patients With Chronic Systolic Heart Failure (ULTIMATE-SHF). Available online: https://clinicaltrials.gov/ct2/show/NCT01646515
- Phosphodiesterase Type 5 Inhibition With Tadalafil Changes Outcomes in Heart Failure (PITCH-HF). Available online: https://clinicaltrials.gov/ct2/show/NCT01910389
- Fernandes AM, Andrade AC, Barroso ND, et al. The Immediate Effect of Sildenafil on Right Ventricular Function in Patients with Heart Failure Measured by Cardiac Magnetic Resonance: A Randomized Control Trial. PLoS One 2015;10:e0119623. [Crossref] [PubMed]
- Bonderman D, Ghio S, Felix SB, et al. Riociguat for Patients With Pulmonary Hypertension Caused by Systolic Left Ventricular Dysfunction: A Phase IIb Double-Blind, Randomized, Placebo-Controlled, Dose-Ranging Hemodynamic Study. Circulation 2013;128:502-11. [Crossref] [PubMed]
- Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute Hemodynamic Effects of Riociguat in Patients With Pulmonary Hypertension Associated With Diastolic Heart Failure (DILATE-1). Chest 2014;146:1274-85. [Crossref] [PubMed]
- Pharmacodynamic Effects of Riociguat in Pulmonary Hypertension and Heart Failure With Preserved Ejection Fraction (DYNAMIC). Available online: https://clinicaltrials.gov/ct2/show/NCT02744339
- Lewis GD, Shah R, Shahzad K, et al. Sildenafil Improves Exercise Capacity and Quality of Life in Patients With Systolic Heart Failure and Secondary Pulmonary Hypertension. Circulation 2007;116:1555-62. [Crossref] [PubMed]
- de Groote P, El Asri C, Fertin M, et al. Sildenafil in heart transplant candidates with pulmonary hypertension. Arch Cardiovasc Dis 2015;108:375-84. [Crossref] [PubMed]
- Pons J, Leblanc MH, Bernier M, et al. Effects of chronic sildenafil use on pulmonary hemodynamics and clinical outcomes in heart transplantation. J Heart Lung Transplant 2012;31:1281-7. [Crossref] [PubMed]
- Hefke T, Zittermann A, Fuchs U, et al. Bosentan Effects on Hemodynamics and Clinical Outcome in Heart Failure Patients with Pulmonary Hypertension Awaiting Cardiac Transplantation. Thorac Cardiovasc Surg 2012;60:26-34. [Crossref] [PubMed]
- Coletta AP, Cleland JG. Clinical trials update: highlights of the scientific sessions of the XXIII Congress of the European Society of Cardiology--WARIS II, ESCAMI, PAFAC, RITZ-1 and TIME. Eur J Heart Fail 2001;3:747-50. [Crossref] [PubMed]
- Kaluski E, Kobrin I, Zimlichman R, et al. RITZ-5: randomized intravenousTeZosentan (an endothelin-A/B antagonist)for the treatment of pulmonary edema. J Am Coll Cardiol 2003;41:204-10. [Crossref] [PubMed]
- Louis A, Cleland JG, Crabbe S, et al. Clinical Trials Update: CAPRICORN, COPERNICUS, MIRACLE, STAF, RITZ-2, RECOVER and RENAISSANCE and cachexia and cholesterol in heart failure. Highlights of the Scientific Sessions of the American College of Cardiology, 2001. Eur J Heart Fail 2001;3:381-7. [Crossref] [PubMed]
- O'Connor CM, Gattis WA, Adams KF, et al. Tezosentan in patients with acute heart failure and acute coronary syndromes: Design of the fourth Randomized Intravenous Tezosentan Study (RITZ-4). Am Heart J 2003;145:S58-9. [Crossref] [PubMed]
- McMurray JJ, Teerlink JR, Cotter G, et al. Effects of Tezosentan on Symptoms and Clinical Outcomes in Patients With Acute Heart Failure. JAMA 2007;298:2009-19. [Crossref] [PubMed]
- Kalra PR, Moon JC, Coats AJ. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol 2002;85:195-7. [Crossref] [PubMed]
- Packer M, McMurray J, Massie BM, et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail 2005;11:12-20. [Crossref] [PubMed]
- Spieker LE, Mitrovic V, Noll G, et al. Acute hemodynamic and neurohumoral effects of selective ETA receptor blockade in patients with congestive heart failure. J Am Coll Cardiol 2000;35:1745-52. [Crossref] [PubMed]
- Givertz MM, Colucci WS, LeJemtel TH, et al. Acute Endothelin A Receptor Blockade Causes Selective Pulmonary Vasodilation in Patients With Chronic Heart Failure. Circulation 2000;101:2922-7. [Crossref] [PubMed]
- Anand I, McMurray J, Cohn JN, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:347-54. [Crossref] [PubMed]
- Clinical Study to Evaluate the Safety and Tolerability of Macitentan in Subjects With Combined Pre- and Post-capillary Pulmonary Hypertension (CpcPH) Due to Left Ventricular Dysfunction (MELODY-1). Available online: https://clinicaltrials.gov/ct2/show/NCT02070991
- Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991;325:1468-75. [Crossref] [PubMed]
- Metra M, Eichhorn E, Abraham WT, et al. Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J 2009;30:3015-26. [Crossref] [PubMed]
- Zimpfer D, Zrunek P, Roethy W, et al. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J Thorac Cardiovasc Surg 2007;133:689-95. [Crossref] [PubMed]
- Kutty RS, Parameshwar J, Lewis C, et al. Use of centrifugal left ventricular assist device as a bridge to candidacy in severe heart failure with secondary pulmonary hypertension. Eur J Cardiothorac Surg 2013;43:1237-42. [Crossref] [PubMed]
- John R, Liao K, Kamdar F, et al. Effects on pre- and posttransplant pulmonary hemodynamics in patients with continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg 2010;140:447-52. [Crossref] [PubMed]
- Mikus E, Stepanenko A, Krabatsch T, et al. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. Eur J Cardiothorac Surg 2011;40:971-7. [PubMed]
- Andrea G, Giuseppe B, Tiziano C, et al. Is fixed severe pulmonary hypertension still a contraindication to heart transplant in the modern era of mechanical circulatory support? A review. J Cardiovasc Med (Hagerstown) 2008;9:1059-62. [Crossref] [PubMed]
- Salzberg SP, Lachat ML, von Harbou K, et al. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg 2005;27:222-5. [Crossref] [PubMed]
- Etz CD, Welp HA, Tjan TD, et al. Medically refractory pulmonary hypertension: treatment with non pulsatile left ventricular assist devices. Ann Thorac Surg 2007;83:1697-705. [Crossref] [PubMed]
- Hamdan R, Mansour H, Nassar P, et al. Prevention of Right Heart Failure After Left Ventricular Assist Device Implantation by Phosphodiesterase 5 Inhibitor. Artif Organs 2014;38:963-7. [Crossref] [PubMed]
- Klodell CT, Morey TE, Lobato EB, et al. Effect of Sildenafil on Pulmonary Artery Pressure, Systemic Pressure, and Nitric Oxide Utilization in Patients With Left Ventricular Assist Devices. Ann Thorac Surg 2007;83:68-71; discussion 71. [Crossref] [PubMed]
- Tedford RJ, Hemnes AR, Russell SD, et al. PDE5A Inhibitor Treatment of Persistent Pulmonary Hypertension After Mechanical Circulatory Support. Circulation: Heart Failure 2008;1:213-9. [Crossref] [PubMed]
- Antoniou T, Prokakis C, Athanasopoulos G, et al. Inhaled Nitric Oxide Plus Iloprost in the Setting of Post-Left Assist Device Right Heart Dysfunction. Ann Thorac Surg 2012;94:792-8. [Crossref] [PubMed]
- LaRue SJ, Garcia-Cortes R, Nassif ME, et al. Treatment of Secondary Pulmonary Hypertension with Bosentan after Left Ventricular Assist Device Implantation. Cardiovasc Ther 2015;33:50-5. [Crossref] [PubMed]
- Clinical Study to Assess the Efficacy and Safety of Macitentan in Patients With Pulmonary Hypertension After Left Ventricular Assist Device Implantation (Soprano). Available online: https://clinicaltrials.gov/ct2/show/NCT02554903


