Pupillary abnormalities in non-selected critically ill patients: an observational study
Introduction
Pupillary examination is a key element of neurologic surveillance in critically ill patients (1). The size and reactivity to light of each pupil allows an exploration of brainstem function and should be recorded periodically in intensive care units (ICU) (2-4). Indeed, pupillary abnormalities (PAs), i.e., anisocoria and/or pupillary areflexia, may represent a sign of acute brain damage, which is particularly informative in comatose patients (1-4). In neurosurgical patients, PAs are frequent and represent a reliable marker of brain herniation or ischemia (5-8). Surprisingly, in non selected critically ill patients, data on the epidemiology of PAs are scarce. In any event, clinical assessment of brainstem reflexes is recommended in all critically ill patients and pupillary examination is routinely performed in non-neurosurgical ICUs (1,2). The purpose of the present study is to describe the prevalence and the causes of PAs in non-selected ICU patients.
Methods
The study was approved by the ethics committee board of Lyon, France “Comité de Protection des Personnes Lyon Sud-Est II” (CAL No. 2011-016) and was carried out with the ethical standards set forth in the Helsinki Declaration of 1975 (and all subsequent revisions). The need for informed consent was waived in view of the observational nature of the study. All patients (or their relatives) were informed that their data could be used anonymously for academic research.
Type of study and population
Over a 6-month period, we conducted a prospective, observational analysis of data from adult patients admitted into a 15-bed university-affiliated medical ICU. Patients were included if they had at least one pupillary exam during their ICU stay; there was no exclusion criteria.
Data collection and definitions
The following data were collected for all patients at admission: age, sex, history of neurological and ophthalmological disease, reason for ICU admission, Glasgow Coma Score (GCS), number of organ failures according to the ODIN (Organ Dysfunction and/or Infection) score and organ supports. Simplified Acute Physiology Score II (SAPS II), length of ICU stay and vital status at ICU discharge were also recorded. After completing a 1-hour refresh course on pupillary examination provided by trained physicians, the nursing staff performed the pupillary surveillance based on a local protocol. Briefly, pupillary assessment was made in a dimly lighted room upon admission and every 1 to 4 hours, in accordance with medical prescription. The examiner checked the size and equality of patient’s pupils (with both eyes open) and their direct response to a bright light. To increase inter-observer agreement, pupil gauge was used systematically to measure pupil size. In the same way, a single model of penlight (Adlight II, ADC, NY, USA) was used to test light reactivity throughout the study. Nurses reported pupil sizes and reactivity on a computer-based data acquisition system supported by IntelliSpace Critical Care and Anaesthesia (ICCA) software (Philips Medical System, the Netherlands). When a nurse detected a new PA, the physician responsible for the patient was systematically informed without any delay.
PA was defined by the presence of anisocoria and/or areflexia at ICU admission or by occurrence during the ICU stay. As previously described, anisocoria was defined by a difference of 1 mm or more between the size of the two pupils and pupillary areflexia (nonreactive pupil) as the absence of visible pupil constriction to light stimulation. If a patient presented a new episode of PA after a sustained period of recovery (>24 hours), it was recorded as an independent PA.
In the case of confirmed PA, physicians sought to determine the cause based on medical history, clinical examination, complementary exams and analysis of the patient’s treatments. The choice of diagnosis procedure was left to the discretion of the physicians. The final etiologies of PA were determined through review of patients’ charts and outcomes.
Statistical analysis
Results are expressed as mean ± SD for quantitative variables, and number (%) for qualitative data. Comparisons were performed using Fisher’s exact test for categorical data and Mann-Whithney U test for continuous variables, as appropriate. Statistical significance was defined as a P value <0.05. Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Sofware, La Jolla, CA, USA).
Results
We included 297 patients who had an average of 6±9 pupillary examinations per day, totaling 11,360 pupillary assessments. Baseline characteristics of patients are reported in Table 1. During the ICU stay, 109 patients (37%) presented at least one PA. The first episode of PA was diagnosed upon ICU admission in 59 patients (20%) and 95±123 hours after admission in 50 patients (17%). As shown in Table 1, patients with PA had significantly more organ failures, higher SAPS II score, longer ICU stays and a higher mortality rate than patients without PA (P<0.05).
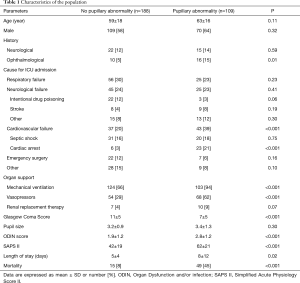
Full table
As shown in Table 2, 128 PAs were recorded, of which 59 (46%) were present upon ICU admission and 83 (65%) were transient. A maximum of 2 PAs were observed in a same patient. In 8 (6%) cases, the PA was known before ICU admission (Table 2) and was due to blindness or cataract surgery. Areflexia was the most common PA (n=78, 61%) followed by anisocoria (n=33, 26%) and association of anisocoria with unilateral or bilateral areflexia (n=17, 23%) (Table 2). At least one neurological complementary exam (e.g., brain imaging or electroencephalogram) was performed for 48 (38%) PAs. PAs were associated with ischemia-induced structural brain lesions in 46 cases (36%), and were mainly related to cardiac arrest and stroke (n=34, 74%) (Table 2). As expected, the combination of areflexia and anisocoria was significantly (P<0.05) more frequently associated with an organic lesion (n=11/17, 65%) than areflexia or anisocoria alone (n=35/111, 32%) (Table 2). Moreover, the combination of anisocoria with bilateral areflexia was associated with an organic lesion in all cases (n=9/9, 100%).
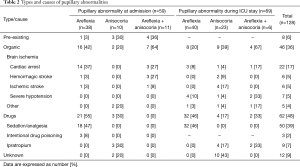
Full table
Most of the PAs were attributed to drugs (n=62, 48%); 50 (39%) were bilateral pupillary areflexia (pupillary diameter: 2.1±0.7) due to general anesthesia with sufentanil and midazolam (Table 2). One case of anisocoria was a transitory Bernard-Horner syndrome following internal jugular vein cannulation under local anesthesia. For 12 (9%) PAs, all reactive anisocoria, the etiology remained unknown. Interestingly, Ipratropium nebulization via face-mask in non-ventilated patients with chronic obstructive pulmonary disease exacerbation led to 9 (7%) cases of transient anisocoria. Among these patients, 4 (44%) were comatose (GCS <6) and 5 (56%) were awake (GCS ≥14) at the time of pupillary examination (performed within 1 hour after Ipratropium nebulization).
Discussion
The present study, reporting a high prevalence of PAs frequently associated with brain organic lesions or drug side effects, highlights the clinical interest of the pupillary surveillance in non-selected critically ill patients.
Our results showed that more than one third of non-selected critically ill patients experienced PA during their ICU stay. To the best of our knowledge, no previous study has reported the prevalence of PAs in this population. Our findings should be cautiously compared with those from previous studies that almost uniquely included neurosurgical patients (4-9). The prevalence of PAs may vary according to the quality of the pupillary evaluation, which can be challenging at the bedside (2,10-14). Indeed, the difference in pupillary diameter between the two eyes should be measured under equal illumination and with vergence and accommodation (2). Obviously, this is rarely the case in ICUs. Moreover, clinical examinations performed with a penlight are often unable to detect a light reflex when pupils constrict less than 0.3 mm and/or when pupils are very small (10,12). This scenario might be very frequent in ICUs, notably because of the routine use of morphine derivatives, which promote the occurrence of miosis (15). Indeed, several reports indicate that opioids given at anesthetic doses likely result in a decreased pupillary light constriction below the threshold of 0.3 mm (12,16,17). Therefore, we cannot rule out the fact that some of areflexia were false positives in our study. Some PAs could also have been missed because of inadequate examinations or monitoring intervals (12,13,17). To date, the best way to limit misdiagnosis of PAs is probably to objectively measure the size and reactivity of pupils by using a hand-held automated pupillometer (11,12,15 17). Unfortunately, this technology is rarely available in non-neurological ICU. This is probably mainly due to the high price of this technology. However, the high frequency of clinical PAs we detected in our study may help to justify the purchase of these devices in general ICUs.
The principle aim of serial pupil examinations in ICUs is to allow early detection of acute brain injury. In trauma patients, acute PA has good accuracy in predicting escalating mass effect (e.g., hematoma, contusion, diffuse brain swelling) (5-8,18). In these patients, implantation of intracranial pressure (ICP) monitors, osmotherapy, and/or neurosurgery are often required to manage intracranial hypertension (11,18). In our study, PAs were associated with structural brain lesions in more than one third of cases. As expected, the causes of PAs differed from those usually reported after traumatic brain injury. In most cases, cardiac arrest and ischemic stroke were responsible for brain damage in our cohort. Usually, invasive procedures (e.g., implantation of ICP sensors or neurosurgery) are not required to manage such medical causes of PAs. Nevertheless, detection of PAs might have significant implications for the care of non-selected critically ill patients. For instance, it would help the early diagnosis and treatment of strokes occurring after ICU admission, especially in sedated patients, when an anisocoria is detected. Moreover, the clinical interest of serial pupillary examinations is not limited to the diagnosis of acute brain injury. Indeed, identification of drug-induced PAs, which was a common finding in the present study, might help clinicians to adapt treatments and/or surveillance. The occurrence of pupillary areflexia in relation to the administration of an anesthetic drug (e.g., opioids) should encourage the reappraisal of the treatment dose and/or the use of an automated pupillometer able to detect a small light reflex missed by clinical examination (11,15). In the present work, anisocoria was observed in 9 awake patients after nebulization of ipratropium bromide. Such an observation should lead to the careful fitting of the nebulizing apparatus in order to avoid direct exposure of the eyes to the drug, which is known to have potent anti-cholinergic effects (19).
In the present study, less than half of the patients with PA had complementary investigations. This contrasts with brain trauma patients, among whom brain imaging is generally performed when PA occurs (18). Although many PAs had obvious causes, the systematic research of the patient’s history and side effects of drugs on pupils allowed us to avoid some unnecessary examinations. Given the aging population, an increasing number of patients have ophthalmic history, which can lead to or favor PAs. Therefore, it would be useful to systematically document pre-existing PAs in the medical record. ICU staffs should also be warned about the risk of anisocoria with atropine-like drugs or the risk of Bernard-Horner syndrome after local anesthesia for internal jugular catheter placement (19,20).
Conclusions
In total, our study confirms the paramount importance of the pupillary surveillance of non-selected critically ill patients. PAs occurred in one third of patients and were mainly associated with acute brain ischemia or adverse drug events. In addition to helping clinicians in the early detection of acute structural brain disorders, repeated pupillary examinations might be of interest in the identification of drug misuse in the ICU. Further research is needed to determine the prognosis value of PAs in this population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics committee board of Lyon, France “Comité de Protection des Personnes Lyon Sud-Est II” (CAL No. 2011-016) and conformed to the provisions of the Helsinki Declaration as revised in 2013. The need for informed consent was waived in view of the observational nature of the study. All patients (or their relatives) were informed that their data could be used anonymously for academic research.
References
- Sharshar T, Citerio G, Andrews PJ, et al. Neurological examination of critically ill patients: a pragmatic approach. Report of an ESICM expert panel. Intensive Care Med 2014;40:484-95. [Crossref] [PubMed]
- Singhal NS, Josephson A. A practical approach to neurologic evaluation in the intensive care unit. J Crit Care 2014;29:627-33. [Crossref] [PubMed]
- Wilhelm H. Neuro-ophthalmology of pupillary function--practical guidelines. J Neurol 1998;245:573-83. [Crossref] [PubMed]
- Tokuda Y, Nakazato N, Stein GH. Pupillary evaluation for differential diagnosis of coma. Postgrad Med J 2003;79:49-51. [Crossref] [PubMed]
- Ritter AM, Muiezlaar JP, Barnes T, et al. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery 1999;44:941-8. [Crossref] [PubMed]
- Hoffmann M, Lefering R, Rueger JM, et al. Pupil evaluation in addition to Glasgow Coma Scale components in prediction of traumatic brain injury and mortality. Br J Surg 2012;99 Suppl 1:122-30. [Crossref] [PubMed]
- Chesnut RM, Gautille T, Blunt BA, et al. The localizing value of asymmetry in pupillary size in severe head injury: relation to lesion type and location. Neurosurgery 1994;34:840-5. [Crossref] [PubMed]
- MRC CRASH Trial Collaborators, Perel P, Arango M, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 2008;336:425-9. [Crossref] [PubMed]
- Daou B, Kent AP, Montano M, et al. Decompressive hemicraniectomy: predictors of functional outcomes in patients with ischemic stroke. J Neurosurg 2016;124:1773-9. [Crossref] [PubMed]
- Larson MD, Muhiudeen I. Pupillometric analysis of the 'absent light reflex'. Arch Neurol 1995;52:369-72. [Crossref] [PubMed]
- Zafar SF, Suarez JI. Automated pupillometer for monitoring the critically ill patient: A critical appraisal. J Crit Care 2014;29:599-603. [Crossref] [PubMed]
- Olson DM, Stutzman S, Saju C, et al. Interrater reliability of pupillary assessments. Neurocrit Care 2016;24:251-7. [Crossref] [PubMed]
- Mojumder DK, Patel S, Nugent K, et al. Pupil to limbus ratio: Introducing a simple objective measure using two-box method for measuring early anisocoria and progress of pupillary change in the ICU. J Neurosci Rural Pract 2015;6:208-15. [Crossref] [PubMed]
- Hoonpongsimanont W, Nguyen K, Deng W, et al. Effectiveness of a 40-minute ophtalmologic examination teaching session on medical student learning. West J Emerg Med 2015;16:721-6. [Crossref] [PubMed]
- Rouche O, Wolak-Thierry A, Destoop Q, et al. Evaluation of the depth of sedation in an intensive care unit based on the photo motor reflex variations measured by video pupillometry. Ann Intensive Care 2013;3:5. [Crossref] [PubMed]
- Larson MD, Kurz A, Sessler DI, et al. Alfentanyl blocks the pupillary dilatation in response to noxious stimulation but does not diminish the light reflex. Anesthesiology 1997;87:849-55. [Crossref] [PubMed]
- Couret D, Boumaza D, Grisotto C, et al. Reliability of standard pupillary practice in neurocritical care: an observational, double-blinded study. Crit Care 2016;20:99. [Crossref] [PubMed]
- Maas AI, Stocchetti N, Bullock R, et al. Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008;7:728-41. [Crossref] [PubMed]
- Bisquerra RA, Botz GH, Nates JL. Ipratropium-bromide-induced acute anisocoria in the intensive care setting due to ill-fitting face masks. Respir Care 2005;50:1662-4. [PubMed]
- Butty Z, Gopwani J, Mehta S, et al. Horner’s syndrome in patients admitted to the intensive care unit that have undergone central venous catheterization: a prospective study. Eye (Lond) 2016;30:31-3. [Crossref] [PubMed]


