A systematic and genome-wide correlation meta-analysis of PD-L1 expression and targetable NSCLC driver genes
Introduction
Targeted therapy and immunotherapy are innovative therapies for non-small cell lung cancer (NSCLC) after traditional chemotherapy and radiotherapy.
Though the later were considered as standard treatment for patients with advanced untargetable NSCLC (1), no more than one third of patients with metastatic NSCLC respond to platinum-based doublet chemotherapy (2). NSCLC is a disease sub-classified by molecular subsets with important driver oncogenes, such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). In NSCLC patients with ALK translocation or EGFR mutation, molecular-targeted therapy is proved to be superior to chemotherapy (3,4), but acquired resistance after treatment initiation develops within 1 to 2 years in the majority of patients (5).
The binding of programmed death 1 (CD279, PD-1) molecules and its ligands PD-L1 and PD-L2 induces inhibitory immune signals, assisting the evasion of host immune response (6), which is immunotarget with therapeutic potential (7). Recently, blocking of the interaction has been reported as a promising strategy for NSCLC treatment (8-10). However, the appropriate combination of PD-1/PD-L1 blockade and the existing anti-cancer therapies are not clear.
High tumor mutational rate appears to contribute to enhanced tumor immunogenicity, indicating that there may be an increased sensitivity to immune checkpoint blockade in these tumors (11). Several studies have reported that the immune checkpoint inhibitor may be more effective in tumors bearing high levels of somatic mutations (10,12). Moreover, in terms of somatic mutation and tumor immunology, whether the gene mutation status related to tumor immune function is still unconfirmed.
Recently, the association between ALK, EGFR, and Kirsten rat sarcoma viral oncogene homolog (KRAS) status and PD-L1 expression are reported (13,14). However, the correlations between druggable NSCLC driver mutations and tumor PD-L1 expression remain inconclusive. Based on these results, a meta-analysis covering the current reported data from correlated studies in combination with a bioinformatic analysis of publicly available RNA sequencing data [from The Cancer Genome Atlas (TCGA)] was conducted to examine a creditable association between PD-L1 and targetable driver genes.
Methods
Search strategy for meta-analysis
All the potential articles in English were searched in the PubMed, Web of science and Embase databases. We use the keywords as: “B7-H1” or “CD274” or “PDL1” or “Programmed cell death 1 ligand 1” and “Programmed death-ligand 1” in combination with “lung cancer”. Then we performed second round of searching by adding driver gene names such as ALK, EGFR, ROS1, MET, KRAS, BRAF, ERBB2, NRAS, PIK3CA or RET. The search was performed for records prior to September 1, 2016. The latest published studies will be used to analyze when the repeated population were included in different publications
Meta-analysis data collection
Included studies were based on the inclusion criteria as: (I) human lung cancer studies; (II) studies which reported the relationship between druggable genes alteration and tumor PD-L1 expression with defined level; (III) published in English. Exclusion criteria were: (I) case reports, conference abstract, editorials, expert opinions, letters, ongoing studies and reviews; (II) studies failed to extract or without usable data; (III) small cell lung cancer type; (IV) duplicate publications.
Data extraction
To find all appropriate researches, two researchers, based on the inclusion criteria, independently searched the available data for meta-analysis. We resolved our disagreements through discussion and consultation. The extraction data included the leading author, publication year, the lung cancer subtype, the patient ethnicity, the PD-L1 expression data, gene mutation state and the clinical variables. We assessed the quality of included studies by the Newcastle-Ottawa scale.
Summary effect analysis
All the analyses were conducted using the Review Manager 5.2. The results were presented in the form of pooled odds ratios (ORs) with 95% confidence interval (CI) and P value (less than 0.05 was considered as statistical significance). The fixed effect mode (I2 values <50%) and the random-effects model (I2 values ≥50%) were used according to the outcome of heterogeneity. Subgroup and sensitivity analysis were stratified for predisposed factors when available.
Publication bias
The potential publication bias was minimized by extensive search strategy. The publication bias was visually assessed by graphical funnel plot. Begg’s test was employed to detect funnel plot asymmetry.
TCGA lung cancer RNA-seq analysis
RNA expression information and the corresponding clinical data of 1,089 lung cancer cases were collected from TCGA level 3 RNA-seq database (http://cancergenome.nih.gov/). These datasets represent the normalized gene expression values of lung adenocarcinoma and lung squamous cancers and include 60,483 discrete genes. The associations of tumor PD-L1 expression and targetable lung cancer driver genes were calculated by Pearson correlation analysis and Z-test. We further used the Wilcoxon signed-rank test to examine whether there is a PD-L1 expression difference between lung adenocarcinoma and lung squamous cancers. Two genes were correlated if the correlation coefficient is more than 0.5.
Results
Literature retrieval results
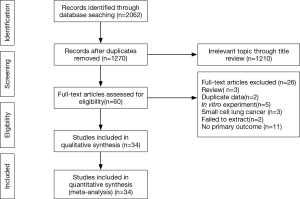
The initial search obtained 1,270 potential datasets from publicly available literature. Of these, 1,210 were excluded since they lacked detailed data regarding PD-L1 and driver genes (expression or mutation). In the remaining 60 literature sources for full-text assessment, 34 were finally included for this meta-analysis. Figure 1 summarizes the selection process.
Studies features
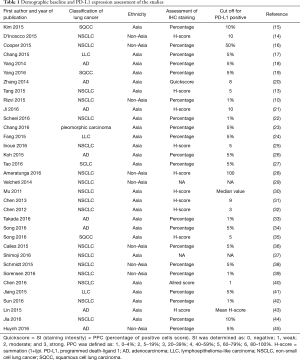
The total number of NSCLC patients was 9,934 cases, of which 6,202 were Asian origin. The expression rate of PD-L1 was 43.4% (95% CI: 37.4–49.6%). In the subgroup analysis, we found the overall PD-L1 expression rate was 45.3% (95% CI: 34.3–56.9%) in squamous cell lung cancer and 41.4% (95% CI: 34.1–49.2%) in adenocarcinoma. In addition, compared with the PD-L1 expression rate by studies from different ethnicities, the Asian population had a higher expression rate of 47.5% (95% CI: 41.8–53.2%) than non-Asians population 34.5% (95% CI: 22.5–48.9%). Table 1 shows the clinical and demographic features of the included studies.
Full table
PD-L1 expression is associated with EGFR expression
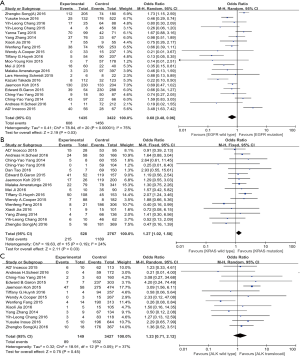
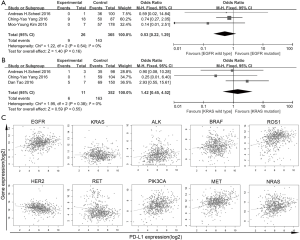
To explore the potential correlation between EGFR mutation and PD-L1 expression, we further subgroup-analyzed data obtained from twenty-one studies with 4,857 patients. Among 1,435 tumors with EGFR mutations, 608 (36.7%) had PD-L1 expression positive status, while 1,456 (44.1%) of 3,422 EGFR wild type tumors showed positive PD-L1 expression. A significant correlation (OR: 0.68, 95% CI: 0.48–0.96; P=0.03) between EGFR wild type and PD-L1 expression was found (Figure 2A).
KRAS mutation is associated with PD-L1 expression
Data extracted from sixteen studies, including a total of 3,295 cases, was analyzed the association between KRAS mutation and PD-L1 expression. In 528 patients with KRAS mutations, 215 (44.6%) patients were positive PD-L1 expressed, while 1,189 (42.2%) of 2,767 wild-type KRAS cases showed positive PD-L1 expression. In this analysis, we reported a significant association (OR: 1.27, 95% CI: 1.02–1.58; P=0.03) between KRAS mutation and PD-L1 expression in clinical dataset (Figure 2B).
The ALK status and PD-L1 expression
In 13 studies, including 3,576 patients, were previously reported the association between ALK translocation and PD-L1 expression. In tumors with ALK translocation, 89 (50.0%) of the 149 tumors specimen had positive PD-L1 expression, while 1,532 (42.8%) of 3,427 ALK wild type tumors stained as PD-L1 positive. In this analysis with large population, the correlation between ALK translocation and PD-L1 expression was unable to detect (OR: 1.23, 95% CI: 0.71–2.12; P=0.45) (Figure 2C).
Correlation between clinical features and PD-L1 expression
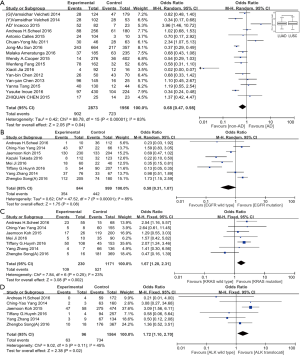
At the end of our literature review, fifteen studies, included 4,829 cases, had reported the association of histological types of lung cancer and PD-L1 expression. The positive expression rate of PD-L1 in adenocarcinoma tumors and non-adenocarcinoma tumors were 902 (33.3%) of 2,873 and 723 (42.5%) of 1,956, respectively. A significant correlation (OR: 0.68, 95% CI: 0.47–0.98; P=0.04) between the expression of PD-L1 and non-adenocarcinoma exists (Figure 3A). Moreover, we interrogated the lung adenocarcinoma and squamous lung cancer genome-wide RNA sequencing dataset of TCGA, consisting of 1,089 cases in total, and found significantly lower PD-L1 expression in lung adenocarcinoma than in lung squamous cancer (P=0.023) (Figure 3A). Similar to the mentioned meta-analysis process, we included thirty-two studies with 8,013 patients for studying the association between gender and PD-L1 expression, no significant association was found (OR: 0.87, 95% CI: 0.73–1.04; P=0.12) (Table 2). Likewise, clinical data including thirty studies with a total of 7,362 cases were used for the assessment of the association between smoking history and PD-L1 expression. However, our results showed that no significant association was indicated (OR: 1.20, 95% CI: 0.95–1.52; P=0.12) (Table 2).

Full table
Subgroup analysis
We conducted further subgroup analysis based on two major lung cancer histology (adenocarcinoma against squamous carcinoma). In the former, ALK translocation and KRAS mutation status is associated with PD-L1 expression (Figure 3B-D, Table 2). However, in lung squamous carcinoma, our results showed that the genetic status of EGFR, KRAS, gender or smoking status had no association with PD-L1 expression (Figure 4A,B, Table 2).
Genome-wide correlation analysis of PD-L1 and druggable genes
To further investigate whether the common NSCLC driver genes (EGFR, KRAS, ALK, MET, ROS1, PIK3CA, RET) were correlated with PD-L1 expression, we analyzed 1,089 patient data sets from TCGA level 3 RNA-seq using Pearson’s correlation coefficient. There were weak or no correlation between PD-L1 expression level and druggable genes expression, ALK (ra=0.149, rs=0.033), BRAF (ra=0.113, rs=−0.05), EGFR (ra=0.251, rs=−0.044), ERBB2 (ra=−0.195, rs=−0.148), KRAS (ra=0.006, rs=0.006), MET (ra=0.361, rs=0.135), NRAS (ra=0.225, rs=−0.093), PIK3CA (ra=0.308, rs=0.115), RET (ra=−0.17, rs=−0.043) or ROS1 (ra=0.295, rs=0.074), in either lung adenocarcinoma (Figure 4C) or squamous cancer (not show).
Discussion
Blocking the binding between PD-L1 and its receptor is a promising therapeutic strategy for cancer immunotherapy, which is a new paradigm for the NSCLC treatment. But none of previous studies extensively explored the association of somatic gene mutation or expression and PD-L1in lung cancer. In our analysis, we found that wild-type EGFR lung cancer patients, KRAS mutation and non-adenocarcinoma histological type were associated with lung cancer PD-L1 expression. However, in mRNA level PD-L1 expression did not correlate with the expression of druggable driver genes.
In this meta-analysis, we found that PD-L1 expression inclined to be associated with wild-type EGFR, but not with EGFR mutation. In previous studies, investigators showed that PD-L1 expressed higher in EGFR-mutant NSCLC cells than in EGFR-wild-type cells (46-48), suggesting that PD-L1 is expressed differently and may function through independent mechanism by EGFR states. In notice, therapeutic effect of anti-PD-1/PD-L1 immunotherapy is associated with the PD-L1 expression level. For example, studies have demonstrated that first-line immunotherapy with pembrolizumab can significantly improve survival in EGFR- or ALK-wildtype and high PD-L1 expressed patients (49). On the contrary, in lung cancer patients with EGFR mutations, the response rate of PD-1 antibodies is low in comparison with wild-type EGFR patients (50-52). For our results revealed that PD-L1 expression is not correlated with EGFR mutation and previous clinical trial showed that PD-1/PD-L1 inhibitors are ineffective in patients with EGFR mutation, it gives us reason to believe that the therapeutic discrepancy is caused by altered immune state after EGFR mutation.
Some studies have reported ALK acts as a regulatory molecule in PD-L1 expression via in vitro experiments (53,54). EML4-ALK can positively regulate PD-L1 expression in NSCLC through activating MEK-ERK and PI3K-AKT signaling pathways (53,55). Koh et al. further proved that EML4-ALK mediated PD-L1 up-regulation in pulmonary adenocarcinoma (26). However, our results showed that no significant correlation in clinical samples between ALK translocation and PD-L1 exists. There may be several reasons for this seeming discrepancy. Firstly, compared to clinical samples with heterogenicity, in-vitro studies were limited to monoclonal cell lines culture. Secondly, the presented study was performed on a large-scale collection of clinical data with varied sample size, baseline clinical characteristics and the definition of positive PD-L1 expression. Even if PD-L1 expression can affect the function ALK expression and EML4-ALK, or vice versa, following our negative results obtained from large-scale comprehensive analysis, the heating prospect of PD-1/PD-L1 inhibitor combined with ALK multi-target protein kinase inhibitor is insecure.
To analyze the association between KRAS gene mutation and PD-L1 expression, we found that KRAS gene mutation is related to PD-L1 expression, suggesting that PD-L1 overexpression could be driven or activated by KRAS gene mutation. This indicates that patients with KRAS gene mutations may be beneficial from PD-1/PD-L1 blockade. Further clinical trials are warranted for this seemingly therapeutic alternative.
As shown by our meta-analysis, we observed a significant PD-L1 expression difference between lung adenocarcinoma and non-adenocarcinoma lung cancer. We also observed, via TCGA RNA-seq data, that PD-L1 expression was significantly lower in patients with adenocarcinoma than in those with lung squamous cell carcinoma (P=0.023). Clinical data indicate that patients with squamous tumors and who were administrated with immunotherapy had higher overall responses rates (ORR) than patients with non-squamous tumors (56). It provides evidence that a potential biomolecular mechanism exists for the explanation of different ORR and PD-L1 expression level in the two major types of lung cancer.
From the current literature, we reported that the presence of EGFR wild-type, KRAS mutations and pulmonary non-adenocarcinoma were associated with expression of PD-L1. While the correlations of PD-L1 and other druggable driver genes are limited. This suggests that for certain subset of lung cancer, PD-1/PD-L1 blockade may have the priority of use as a first line therapeutic strategy, which consisted to the up-to-date concept that higher PD-L1 expression, greater clinical benefit in lung cancer patients (57,58). Though we are the first to apply bioinformatic analysis and meta-analysis to answer whether the current targetable NSCLC driver genes associated with PD-L1 expression, further researches from bench to bed are warranted to verify the benefit of anti-PD-1/PD-L1 combined with modern anti-cancer strategies prior to its extensive clinical applications.
Acknowledgements
Funding: This study was supported in part by the National Natural Science Foundation of China (81672270), Guangdong Province Natural Science Foundation (2015A030313474) and Key project of Guangzhou Science Technology and Innovation committee (201707020042).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Gurubhagavatula S, Liu G, Park S, et al. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J Clin Oncol 2004;22:2594-601. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Tan DS, Yom SS, Tsao MS, et al. The International Association for the Study of Lung Cancer Consensus Statement on Optimizing Management of EGFR Mutation-Positive Non-Small Cell Lung Cancer: Status in 2016. J Thorac Oncol 2016;11:946-63. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 2012;18:6580-7. [Crossref] [PubMed]
- Champiat S, Ferte C, Lebel-Binay S, et al. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014;3:e27817. [Crossref] [PubMed]
- Tang Y, Fang W, Zhang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 2015;6:14209-19. [Crossref] [PubMed]
- D'Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95-102. [Crossref] [PubMed]
- Kim MY, Koh J, Kim S, et al. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 2015;88:24-33. [Crossref] [PubMed]
- Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181-8. [Crossref] [PubMed]
- Chang YL, Yang CY, Lin MW, et al. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer 2015;88:254-9. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer 2016;57:91-103. [Crossref] [PubMed]
- Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014;7:567-73. [Crossref] [PubMed]
- Ji M, Liu Y, Li Q, et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther 2016;17:407-13. [Crossref] [PubMed]
- Scheel AH, Ansen S, Schultheis AM, et al. PD-L1 expression in non-small cell lung cancer: Correlations with genetic alterations. Oncoimmunology 2016;5:e1131379. [Crossref] [PubMed]
- Chang YL, Yang CY, Lin MW, et al. High co-expression of PD-L1 and HIF-1alpha correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 2016;60:125-35. [Crossref] [PubMed]
- Fang W, Hong S, Chen N, et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget 2015;6:33019-32. [Crossref] [PubMed]
- Inoue Y, Yoshimura K, Mori K, et al. Clinical significance of PDL1 and PDL2 copy number gains in nonsmallcell lung cancer. Oncotarget 2016;7:32113-28. [Crossref] [PubMed]
- Koh J, Jang JY, Keam B, et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1alpha and STAT3. Oncoimmunology 2015;5:e1108514. [Crossref] [PubMed]
- Tao D, Han X, Zhang N, et al. Genetic alteration profiling of patients with resected squamous cell lung carcinomas. Oncotarget 2016;7:36590-601. [Crossref] [PubMed]
- Ameratunga M, Asadi K, Lin X, et al. PD-L1 and Tumor Infiltrating Lymphocytes as Prognostic Markers in Resected NSCLC. PLoS One 2016;11:e0153954. [Crossref] [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [Crossref] [PubMed]
- Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682-8. [Crossref] [PubMed]
- Chen YY, Wang LB, Zhu HL, et al. Relationship between programmed death-ligand 1 and clinicopathological characteristics in non-small cell lung cancer patients. Chin Med Sci J 2013;28:147-51. [Crossref] [PubMed]
- Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: A 5-year-follow-up study. Tumori 2012;98:751-5. [PubMed]
- Takada K, Okamoto T, Shoji F, et al. Clinical Significance of PD-L1 Protein Expression in Surgically Resected Primary Lung Adenocarcinoma. J Thorac Oncol 2016;11:1879-90. [Crossref] [PubMed]
- Song Z, Yu X, Cheng G, et al. Programmed death-ligand 1 expression associated with molecular characteristics in surgically resected lung adenocarcinoma. J Transl Med 2016;14:188. [Crossref] [PubMed]
- Song Z, Yu X, Zhang Y. Altered expression of programmed death-ligand 1 after neo-adjuvant chemotherapy in patients with lung squamous cell carcinoma. Lung Cancer 2016;99:166-71. [Crossref] [PubMed]
- Calles A, Liao X, Sholl LM, et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J Thorac Oncol 2015;10:1726-35. [Crossref] [PubMed]
- Shimoji M, Shimizu S, Sato K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer 2016;98:69-75. [Crossref] [PubMed]
- Schmidt LH, Kummel A, Gorlich D, et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One 2015;10:e0136023. [Crossref] [PubMed]
- Sorensen SF, Zhou W, Dolled-Filhart M, et al. PD-L1 Expression and Survival among Patients with Advanced Non-Small Cell Lung Cancer Treated with Chemotherapy. Transl Oncol 2016;9:64-9. [Crossref] [PubMed]
- Chen Z, Mei J, Liu L, et al. PD-L1 expression is associated with advanced non-small cell lung cancer. Oncol Lett 2016;12:921-7. [PubMed]
- Jiang L, Wang L, Li PF, et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. Onco Targets Ther 2015;8:1451-7. [PubMed]
- Sun JM, Zhou W, Choi YL, et al. Prognostic Significance of PD-L1 in Patients with Non-Small Cell Lung Cancer: A Large Cohort Study of Surgically Resected Cases. J Thorac Oncol 2016;11:1003-11. [Crossref] [PubMed]
- Lin C, Chen X, Li M, et al. Programmed Death-Ligand 1 Expression Predicts Tyrosine Kinase Inhibitor Response and Better Prognosis in a Cohort of Patients With Epidermal Growth Factor Receptor Mutation-Positive Lung Adenocarcinoma. Clin Lung Cancer 2015;16:e25-35. [Crossref] [PubMed]
- Jia X, Zhang L, Wu W, et al. Driver Mutation Analysis and PD-L1 Expression in Synchronous Double Primary Lung Cancer. Appl Immunohistochem Mol Morphol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Huynh TG, Morales-Oyarvide V, Campo MJ, et al. Programmed Cell Death Ligand 1 (PD-L1) Expression in Resected Lung Adenocarcinomas: Association with Immune Microenvironment. J Thorac Oncol 2016;11:1869-78. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935-40. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Haratani K, Hayashi H, Tanaka T, et al. Tumor Immune Microenvironment and Nivolumab Efficacy in EGFR Mutation-Positive Non-Small Cell Lung Cancer Based on T790M Status after Disease Progression During EGFR-TKI Treatment. Ann Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Hong S, Chen N, Fang W, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology 2015;5:e1094598. [Crossref] [PubMed]
- Lee MH, Yanagawa J, Rui L, et al. Increased PD-L1 expression in KRAS mutated premalignant human bronchial epithelial cells is enhanced by LKB1 loss and mediated by ERK activation. J Immunother Cancer 2015;3:305. [Crossref]
- Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:4014-21. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]