Expression of redox sensing factor Nrf2 in lung macrophages and type II pneumocytes as a prognostic factor in pneumothorax recurrence
Introduction
Primary spontaneous pneumothorax (PSP) is a common disease, usually occurs in young adolescent males without known underlying lung disorder, and is frequently encountered in clinical practice (1–7% of all pulmonary diseases) (1). PSP occurs in healthy persons without previous trauma or precipitating cause, while secondary spontaneous pneumothorax occurs as a complication of clinically apparent preexisting lung diseases such as chronic obstructive pulmonary disease (COPD) (2). The occurrence rate is reported to be about 18–28/100,000/year for men and 1.2–6/100,000/year for women (1). Most PSP recurrences occur in six months to two years after the initial episode (2). The PSP recurrence rate is 16% to 52% on the ipsilateral side and has been shown to increase with the frequency of episodes (2,3).
PSP is usually caused by chronic destruction of subpleural blebs or bullae, due to imbalance of proteases and anti-proteases, chronic inflammation, hypoxia, and oxidative stress (4). Although PSP usually occurs in patients without clinically apparent lung disorder, 85% to 90% of patients with PSP show localized apical emphysema-like changes on chest computed tomography (CT) scan (4). The formation of ELCs in PSP is associated with elastolysis, the degradation of elastic fibers, which is caused by an imbalance between oxidants and antioxidants, in which lung macrophages and type II pneumocytes play a crucial role (5).
Nuclear factor erythroid 2-related factor 2 (Nrf2) belongs to the Cap‘n’collar (CNC)-basic region leucine zipper (bZIP) transcription factor family (6). It is relatively abundantly expressed in tissues such as the intestine, lung, and kidney where detoxification reactions occur routinely (6). Under normal conditions, Nrf2 is sequestered in the cytosol and bound to the actin cytoskeleton by Kelch-associated protein 1, a cytosolic regulatory protein. When oxidative stress occurs, Nrf2 translocates into the nucleus and binds to the promoter sequence of antioxidant genes (7). In some pulmonary diseases, Nrf2 plays a cytoprotective role by induction of a suite of various antioxidants and phase II enzymes to restore redox homeostasis (7). Previous studies reported that PI3K/AKT, through Nrf2 and its target genes (heme oxygenase 1 and NADPH quinone oxidoreductase), suppressed hyperoxia-induced acute lung injury in lung epithelial cells and alveolar macrophages as well as in mice model (8,9).
In lung diseases, macrophages and type II pneumocytes play crucial roles in the oxidative stress response (10,11). However, no report is available on Nrf2 expression in lung macrophages or type II pneumocytes in PSP patients and their relation with recurrence of PSP. In this study, we explored the role of Nrf2 in PSP by studying its expression patterns in macrophages and type II pneumocytes and then correlated its expression with clinical characteristics and incidence-of-recurrence in a 2-year follow-up period.
Methods
Study cohort
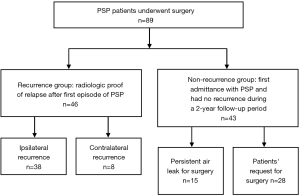
From January 2012 to December 2013, a total of 89 patients with PSP diagnosed by chest radiography and CT scan as well as patient age ≤40 years without evidence of clinical lung disease were included and retrospectively reviewed in this study. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-20130268) and the informed consent was obtained from all the participants. Exclusion criteria included patients with secondary spontaneous pneumothorax, traumatic or iatrogenic pneumothorax, clinical lung diseases such as COPD, pneumonia, asthma, and lung cancer, or lack of medical records. They received surgery owing to recurrence, persistent air leak (for ≥5 days) or patients’ request for preventing future recurrence. The recurrence group was defined as patients with radiological confirmation of ipsilateral or contralateral recurrence of pneumothorax after the first episode of PSP. The non-recurrence group was defined as patients who underwent surgery at their first admittance with PSP and had no recurrence in the 2-year follow-up period. PSP specimens with blebs and adjacent normal lung tissues were obtained from patients undergoing needlescopic video-assisted thoracoscopic surgery (NVATS) at the Division of Chest Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Taiwan. All PSP patients then received regular postoperative follow-up in our outpatient clinic for 2 years.
Surgical technique
The technique of NVATS and mechanical pleurodesis has been described previously (12). We performed three ports NVATS wedge resection and mechanical pleural abrasion for pleurodesis in all the patients.
Immunohistochemistry (IHC)
The procedure of IHC staining was described previously (13). Nrf2 (1:200 dilution, GeneTex, Irvin, CA, USA), CD68 (1:1,000 dilution, Leica, Newcastle, UK), and thyroid transcription factor-1 (TTF-1, 1:300 dilution, Leica, Newcastle, UK) antibodies were used in this study. Negative control with non-addition of Nrf2 antibody was conducted in parallel with the IHC procedure.
Slide evaluation
Nrf2 staining was scored according to the percentage of positively stained cells, determined by two independent experts who were blinded to clinical outcome under the same conditions. Four visual fields were evaluated randomly for each specimen and the staining percentage was stratified into quartiles (≤25% positive cells, 26–50% positive cells, 51–75% positive cells, and ≥76% positive cells). Positive cells less than 75% were categorized as low expression, while positive cells more than 75% were categorized as high expression for statistical analysis.
Statistical analysis
The correlations of Nrf2 expression with clinical characteristics were calculated by the chi-square test or Fisher’s exact test. Student’s t-test was used for comparison between two groups. Incidence-of-recurrence curves were generated using Kaplan–Meier estimates and the significance of differences between curves was evaluated by the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) from univariate and multivariable Cox proportional hazards regression models were used to analyze associations between incidence-of-recurrence and clinicopathological characteristics. All statistical analyses were performed using the SPSS statistical software for PC (SPSS, Chicago, IL, USA) and P values less than 0.05 were considered statistically significant.
Results
Clinical characteristics of patients with PSP
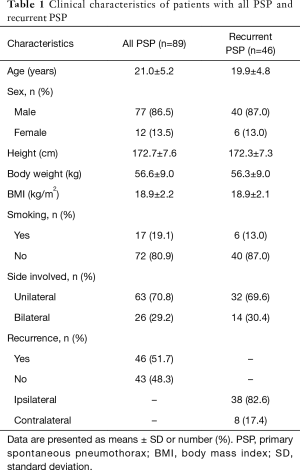
Clinical characteristics of the PSP and recurrent PSP patients were summarized in Table 1. Eighty-nine patients, 77 males and 12 females, with PSP were included in this study. The mean age of the patients was 21.0±5.2 years. In the recurrence group, there were 40 males and 6 females and the mean age was 19.9±4.8 years. About 82.6% (n=38) of the recurrence cases was in the ipsilateral recurrence group, while 17.4% (n=8) was in the contralateral recurrence group (Table 1). As shown in Figure 1, in the cohort of 89 PSP patients, 51.7% of the patients (46 out of 89) underwent surgery owing to recurrence, while 48.3% of the patients (43 out of 89) received surgery owing to persistent air leak (n=15) or patient preference (n=28).
Full table
The expression level of Nrf2 in alveolar macrophages and type II pneumocytes of PSP patients
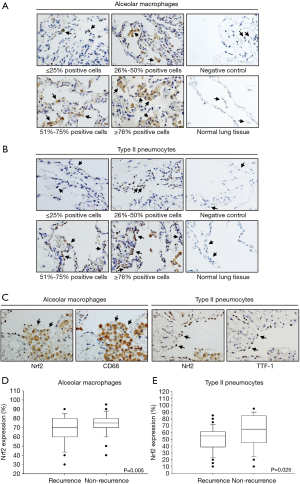
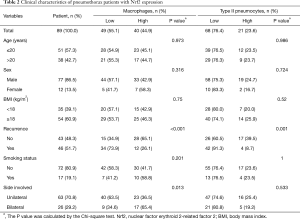
The expression of Nrf2, determined by IHC, was predominantly observed in PSP tissues but not in the adjacent normal lung tissues (Figures 2A,B). Nrf2 was not expressed in alveolar macrophages and type II pneumocytes of the adjacent normal lung tissues from thirteen PSP patients we analyzed. PSP tissues without addition of the Nrf2 antibody performed in parallel with the IHC procedure were used as the negative control. In this study, CD68 and TTF-1 antibodies were applied to recognize alveolar macrophages and type II pneumocytes respectively, and the results confirmed that Nrf2-stained cells were indeed alveolar macrophages or type II pneumocytes of pneumothorax tissues (Figure 2C). As shown in Table 2, 44.9% (macrophages) and 23.6% (type II pneumocytes) of the PSP tissues exhibited high Nrf2 expression, while 55.1% (macrophages) and 76.4% (type II pneumocytes) of the PSP tissues exhibited low Nrf2 expression. Next, we examined whether there were any differences in Nrf2 expression between PSP recurrence group and non-recurrence group. There were significant differences in Nrf2 expression levels between recurrence and non-recurrence groups (alveolar macrophages: P=0.005, Figure 2D; type II pneumocytes: P=0.025, Figure 2E).
Full table
The correlation between the expression pattern of Nrf2 and clinicopathological characteristics of PSP patients
The expression level of Nrf2 in PSP was correlated with clinical characteristics (Table 2). High Nrf2 expression in macrophages of PSP tissues was significantly correlated with decreased recurrence risk and bilateral lesion location (P<0.001 and P=0.013, respectively), while that in type II pneumocytes was only correlated with decreased recurrence risk (P=0.001, Table 2). Age, sex, body mass index (BMI), and smoking status were not found to be significantly correlated with Nrf2 expression. Further analysis in male PSP patients revealed decreased disease recurrence and bilateral lesion location in the group with high Nrf2 expression in macrophages (recurrence: P=0.001; side involved: P=0.019, Table 3). Only disease recurrence was significantly decreased in the group with high Nrf2 expression in type II pneumocytes (P=0.003, Table 3).
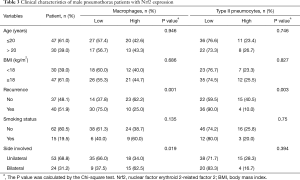
Full table
Relationship between Nrf2 expression in PSP tissues and incidence-of-recurrence in Patients
To investigate the relationship between Nrf2 expression and clinicopathological parameters with incidence-of-recurrence, univariate and multivariable Cox regression analyses were performed (Table 4). In the univariate analysis, Nrf2 expression in macrophages (P=0.005) and Nrf2 expression in type II pneumocytes (P=0.008) showed statistically significant associations with incidence-of-recurrence. In the multivariate analyses, statistically significant relationships with incidence-of-recurrence were observed for three parameters: age (P=0.030), Nrf2 expression in macrophages (P=0.029), and Nrf2 expression in type II pneumocytes (P=0.022).
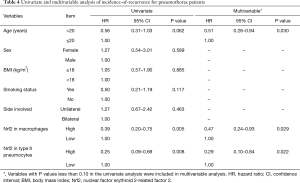
Full table
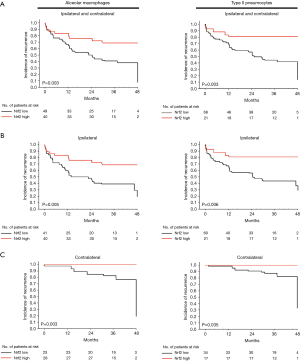
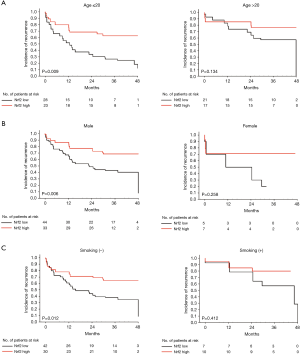
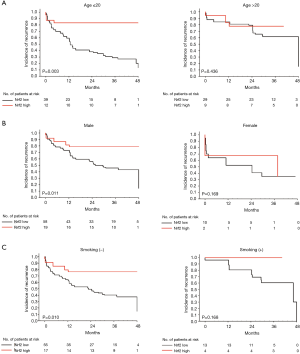
Using Kaplan–Meier estimates, we observed that high Nrf2 expression in macrophages and type II pneumocytes correlated significantly with a better incidence-of-recurrence (P=0.003 and P=0.003, respectively, Figure 3A). High Nrf2 expression in macrophages and type II pneumocytes were also significantly correlated with better ipsilateral or contralateral incidence-of-recurrence (Figures 3B,C). Moreover, incidence-of-recurrence was significantly better for patients with high Nrf2 expression in alveolar macrophages in the age ≤20 (P=0.009, Figure 4A), male (P=0.006, Figure 4B), and non-smoking groups (P=0.012, Figure 4C). Similar results were observed for patients with high Nrf2 expression in type II pneumocytes in the age ≤20 (P=0.003, Figure 5A), male (P=0.011, Figure 5B), and non-smoking groups (P=0.010, Figure 5C). Incidence-of-recurrence was no significant difference for patients with high compared to low Nrf2 expression in the age ≥20, female, and smoking groups (alveolar macrophages, Figure 4; type II pneumocytes, Figure 5).
Discussion
PSP is a common but troublesome thoracic disorder due to its high possibility of unpredictable recurrence and even occurrence on the other side. The basic principles of PSP management include pleural air evacuation, reexpansion of the lung and prevention of recurrence. Without adequate surgical intervention, the recurrence rate on the ipsilateral side is about 25% after the first pneumothorax episode, and 60% to 80% after the second episode (2,3). Many reports have recommended early operation to prevent the recurrence and repeat admissions (14,15). However, there are no available biomarkers to predict recurrence of PSP after different interventions. To the best of our knowledge, this is the first report suggesting the influence of Nrf2 on incidence-of-recurrence of PSP including patients with ipsilateral and contralateral recurrences of PSP (Figure 3).
In the lung tissues, the major sources of Nrf2 are airway epithelial cells, type II alveolar pneumocytes, and alveolar macrophages (8,10,11). However, there have been only few reports investigating the expression of Nrf2 in PSP lung tissue specimens or its correlation with clinical characteristics of PSP patients (16). In this study, we showed that Nrf2 was expressed in alveolar macrophages and type II pneumocytes (Figure 2), the two major cell types that are essential for removing the injured lung tissues and reconstructing the intact alveolus.
In this study, Nrf2 staining by IHC was scored according to the percentage of positive cells. Our results were divided into four quartiles and the cutoff value was defined according to a validated method in previous studies (17,18). Another method for Nrf2 measurement is based on the staining intensity of positively stained cells in the lung tissue of PSP patients. However, the intensity of Nrf2 staining was quite similar among the cases included in the current study. Therefore, only percentage of positive cells was used for the scoring of Nrf2 staining in this study.
Oxidative stress is involved in the predisposing effects of smoking on PSP because cigarette smoke contains a high oxidative burden (16). Exposure to cigarette smoke causes more severe emphysema-like symptoms, and is associated with oxidative DNA adduct formation, apoptosis, and elevated Nrf2 expression (5,10). Nrf2 has an essential protective role in the lungs against oxidative airway diseases, by controlling the expression of antioxidant response element-regulated antioxidant and cytoprotective genes (7). However, the present study showed no significant correlation between smoking status and the expression level of Nrf2 in PSP patients (Table 2). In fact, the negative result is possibly caused by the small number of smokers with PSP (17 out of 89) and smokers with recurrent PSP (6 out of 46) included in our study. Further large scale clinical studies are required to determine whether the expression level of Nrf2 could be a cytoprotective factor against oxidative stress response in smokers with PSP. Interestingly, Nrf2 expression levels in alveolar macrophages and type II pneumocytes were significantly correlated with incidence-of-recurrence in non-smoking patients, but not in smoking patients (Figures 4,5). Nevertheless, high Nrf2 expression in macrophages and type II pneumocytes may exert its protective effect in the patients with PSP.
In agreement with previous reports, we found that most of the PSP patients were male (Table 1). Analysis in male but not in female patients (data not shown) revealed that recurrence rate was significantly different between low and high Nrf2 expression groups (Table 3). Further analysis of PSP patients showed a significant difference in incidence-of-recurrence in males with low versus high Nrf2 expression (Figures 4,5). The lack of association in the female group may be due to the small sample size in this study.
Some factors, such as patient’s status (age or BMI), have been found to be involved in recurrent spontaneous pneumothorax (3). The mean age of our recurrent PSP patients was less than 20 years (Table 1). In the univariate analysis, the HR of incidence-of-recurrence in the age >20 years group was 0.56 and that in the age ≤20 years group was 1.0 (Table 4), revealing that recurrence predominantly affects young patients with PSP. We also found that the adjusted HR for recurrence in patients with high Nrf2 expression in macrophages and type II pneumocytes were 0.47 and 0.29, respectively (Table 4). Furthermore, high Nrf2 expression in alveolar macrophages and type II pneumocytes were correlated with better incidence-of-recurrence in the age ≤20 group (Figures 4,5). Therefore, identification of protective factors in young PSP patients, including Nrf2 in this study, may provide useful information for physicians to design better schemes for prevention of PSP recurrence. Further clinical correlation of Nrf2 with oxidative stress marker 4-hydroxynonenal (4-HNE) (16) should be evaluated to determine whether the expression levels of Nrf2 could be a protective factor against oxidative stress in PSP patients.
Of note, the rates of PSP recurrence were high in our study, with 51.7% of patients having recurrent PSP after the first episode of PSP. One reason for this phenomenon was due to the fact that most of the recurrent PSP patients who were primarily treated at nearby local hospitals were referred to our medical center for surgical management. In fact, our post-operative recurrence rate of PSP is about 4.3%, lower than the average (10% or less) (1). Therefore, the high PSP recurrence rate was possibly due to the different therapeutic procedures being used for patients including observation, needle aspiration, and tube thoracostomy at local hospitals before coming to our medical center.
In conclusion, we observed a negative correlation between Nrf2 expression in PSP tissues and the recurrence risk in PSP patients. High Nrf2 expression level was found to be an independent predictor for better recurrence-free survival in PSP. Our results indicate a potential protective role of Nrf2 in reducing the risk of PSP recurrence.
Acknowledgements
Funding: This work was supported by grants from the Kaohsiung Medical University Hospital (KMUH102-2M19, KMUH102-2M21) and Kaohsiung Municipal Ta-Tung Hospital (kmtth-104-013, kmtth-105-048) of Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-20130268) and the informed consent was obtained from all the participants.
References
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [Crossref] [PubMed]
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005;10:378-84. [Crossref] [PubMed]
- Grundy S, Bentley A, Tschopp JM. Primary spontaneous pneumothorax: A diffuse disease of the pleura. Respiration 2012;83:185-9. [Crossref] [PubMed]
- Wallaert B, Gressier B, Marquette CH, et al. Inactivation of alpha 1-proteinase inhibitor by alveolar inflammatory cells from smoking patients with or without emphysema. Am Rev Respir Dis 1993;147:1537-43. [Crossref] [PubMed]
- Itoh K, Chiba T, Takahashi S, et al. An nrf2/small maf heterodimer mediates the induction of phase ii detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 1997;236:313-22. [Crossref] [PubMed]
- Abed DA, Goldstein M, Albanyan H, et al. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents. Acta Pharm Sin B 2015;5:285-99. [Crossref] [PubMed]
- Papaiahgari S, Zhang Q, Kleeberger SR, et al. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal 2006;8:43-52. [Crossref] [PubMed]
- Reddy NM, Potteti HR, Vegiraju S, et al. PI3K-AKT signaling via Nrf2 protects against hyperoxia-induced acute lung injury, but promotes inflammation post-injury independent of Nrf2 in mice. PLoS One 2015;10:e0129676. [Crossref] [PubMed]
- Goven D, Boutten A, Lecon-Malas V, et al. Prolonged cigarette smoke exposure decreases heme oxygenase-1 and alters Nrf2 and Bach1 expression in human macrophages: roles of the MAP kinases ERK(1/2) and JNK. FEBS Lett 2009;583:3508-18. [Crossref] [PubMed]
- Messier EM, Bahmed K, Tuder RM, et al. Trolox contributes to nrf2-mediated protection of human and murine primary alveolar type ii cells from injury by cigarette smoke. Cell Death Dis 2013;4:e573. [Crossref] [PubMed]
- Chou SH, Li HP, Lee JY, et al. Needlescopic video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2009;18:221-4. [Crossref] [PubMed]
- Hung AC, Lo S, Hou MF, et al. Extracellular visfatin-promoted malignant behavior in breast cancer is mediated through c-Abl and STAT3 activation. Clin Cancer Res 2016;22:4478-90. [Crossref] [PubMed]
- Ben-Nun A, Soudack M, Best LA. Video-assisted thoracoscopic surgery for recurrent spontaneous pneumothorax: the long-term benefit. World J Surg 2006;30:285-90. [Crossref] [PubMed]
- Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: A systematic review of randomised and non-randomised trials. Lancet 2007;370:329-35. [Crossref] [PubMed]
- Goven D, Boutten A, Lecon-Malas V, et al. Induction of heme oxygenase-1, biliverdin reductase and h-ferritin in lung macrophage in smokers with primary spontaneous pneumothorax: Role of hif-1alpha. PLoS One 2010;5:e10886. [Crossref] [PubMed]
- Lee YC, Chen YJ, Wu CC, et al. Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol Oncol 2012;125:742-50. [Crossref] [PubMed]
- Cheng YJ, Lee YC, Chiu WC, et al. High id1 expression, a generally negative prognostic factor, paradoxically predicts a favorable prognosis for adjuvant paclitaxel plus cisplatin therapy in surgically treated lung cancer patients. Oncotarget 2014;5:11564-75. [Crossref] [PubMed]