New insights in non-small-cell lung cancer: circulating tumor cells and cell-free DNA
Introduction
Lung cancer is currently the malignant tumor with the highest mortality rate in both sexes worldwide, with 1.8 newly diagnosed cancer cases and 1.6 million cancer related-deaths every year (1). Despite the significant advances made in both diagnostic and therapeutic approaches in recent years, mainly the emergence of molecular subsets defined by specific oncogenic aberrations, the overall 5 years survival remains at only 16%, probably due to inadequate screening programs and the late onset of clinical symptoms. All this, means that the diagnosis is made in advanced stages and consequently, patients have a very poor prognosis (1,2).
According to its histopathological features, lung cancer has historically been divided into two main types: 85% are non-small-cell lung cancer (NSCLC) and 15% are small cell lung cancer (SCLC). The NSCLC subtype is divided into adenocarcinomas (ADC), which represents 50% of all cases, and into squamous cell carcinoma (SCC). In the past, NSCLC have been lumped together, but this has changed because of the therapeutic implications that have separated histologies (3).
NSCLC is a heterogeneous disease, our view of NSCLC from histopathological descriptions of precise molecular identities has transformed in the past decade. Treatment should be personalized according to the patient’s clinical condition (Performance Status), stage, histological cell type and molecular profile. Smoking cessation should be strongly encouraged in any stage. Accurate staging is vital for treatment options and prognoses according to the Tumor-Node-Metastasis (TNM) classification system by the Association for the Study of Lung Cancer Staging Committee, which updated the eighth edition in 2016 (4). The treatment of choice when the tumor is still localized is surgery conversely, systemic therapy should be offered to all advanced stage patients. Chemotherapy with platinum doublets should be considered in all stage IV NSCLC patients who do not harbor sensitizing mutations to TKI therapy or other actionable drivers (5). Genetic alterations, which are key oncogenic events (driver mutations), have been identified in NSCLC, but only three of these—epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK)-rearrangements and c-ros oncogene 1 (ROS1)—have been approved with a directed systemic therapy. The genetic alterations tested in some centers, mainly in clinical trials for the time being, are RET, HER2, BRAF and MET, and via different techniques—real time PCR, next-generation sequencing (NGS), FISH and IHC (5,6). Driver mutations are found more frequently in adenocarcinoma, never smokers, females and in patients of East Asian ethnicity. EGFR mutations testing are recommended in all patients with advanced NSCLC of a ADC subtype and exceptionally, testing is recommended in patients with a confident diagnosis of SCC, in never/former light smokers. ALK should be tested in the same group of patients. In advanced-stage disease, molecular targeted therapy is the standard-first line treatment for patients with these identified driver mutations (EGFR, ALK and ROS rearrangements), as the sections below indicate.
Data about intratumor heterogeneity and the clonal nature of driver events have become increasingly available in recent years for the purpose of understanding the biology of NSCLC and its relation to clinical outcome (7). This has allowed the segregation of this tumor through the presence of actionable driver oncogenes and the development of targetable treatments, and also through improvements in technological progresses that offer a new molecular landscape of this malignancy (8).
This review summarizes the most relevant molecular mechanism that underlies NSCLC to later discuss the shift in paradigm from a tissue biopsy to a new approach; the liquid biopsy.
EGFR mutations and resistance mechanisms
The first real evidence that a molecular marker could predict treatment outcome in NSCLC was obtained, with the targeted drug that inhibits EGFR, gefitinib, which is a tyrosine kinase inhibitor (TKI). Gefitinib was approved in 2003 by the Food and Drug Administration (FDA) for single-drug therapy for refractory NSCLC. However, tumor responses were observed in only 10–19 percent of patients with chemotherapy refractory NSCLC, but with rapid and profound responses in this subgroup. Some groups have identified specific activating mutations (deletions in exon 19 and substitutions in exon 21), within the tyrosine kinase domain of EGFR as the molecular correlate of these subgroup’s dramatic responses to Gefitinib (9-12). Moreover, when they focused on responders, they found that a large proportion were women, all had adenocarcinoma, many were of Asian ethnicity, and most had either never smoked or smoked very little compared to average NSCLC patients. This implied a change in the paradigm in NSCLC treatment.
The EGFR gene has 28 exons, and exons 18 through to 21 codes for the TK domain of the receptor. Around 45% of the sensitizing mutations to first generation TKIs (gefitinib, erlotinib) are in this region. Within the EGFR mutations, 90% are deletions in exon 19 (E746–A750) and missense mutations in exon 21, which result in an arginine to leucine substitution (L858R) and exon 18 (G719C, G719S, G719A) substitutions, all of those are responsive to therapy (13). However, the exon 20 insertions is also a driver mutation, but no therapy exists to inhibit this mutation. Mutations in exon 20, which are typically located near the C-helix of the tyrosine kinase domain and only account for up to 4% of all EGFR mutations, are associated with resistance to TKIs. The TK is the part of the protein binds with a ligand from outside the cell. It allows the EGFR to signal the cell to grow and survive. EGFR mutations induce pro-survival and anti-apoptotic signals through downstream targets, including phosphatidylinositol-3-kinase (MAPK)/extracellular-signal-regulated kinase (ERK), and janus kinase (JAK)/signal transducer and the activator of transcription (STAT) cascades, whose knotted networks make cells with EGFR mutations inheritably dependent on a functional EGFR for their survival. This renders the cancer cell sensitive to dying when the switch is turned off by a TKI, and explains why this subgroup of patients benefits (2).
Since 2004 numerous clinical trials have demonstrated improvements in NSCLC harbor mutated EGFR when treated with TKIs in progression-free survival (PFS) and objective response rate (ORR) compared to traditional platinum-based chemotherapy (14,15).
The main problem of the prolonged benefit of these drugs is the development of acquired resistance. The two primary mechanisms of resistance to EGFR TKI include a secondary mutation in EGFR (T790M), which blocks the capacity of Erlotinib to inhibit the receptor, and the amplification of MET. Another mechanism that can be co-identified with EFGR-T790M, is the development of a bypass through the reactivation of signal transductions pathways, such as PI3K/AKT, MAPK/ERK, and JAK/STAT pathways (the frequency of any of these cases with acquired resistance is 10–15%) (16,17).
Resistance mechanism to EGFR through the T790M mutation could be primary or acquired to treatment with TKIs. The reported prevalence of primary resistance is 2.9% for T790M (18) and this mechanism represents 50–60% of the cases of acquired resistance to TKIs (19). Second- and third-generation TKIs have been developed and affect a specific spectrum of genetic alterations, including mutations that may mediate resistance against the conventional TKI. Second-generation EGFR TKIs, such as Afatinib and Dacomitinib, can inhibit lung cancer cell lines with the T790M mutation, but the therapeutic dose might prove too toxic for clinical use. Third-generation TKIs selectively target the T790M mutation. Apart from all this progress, this year the FDA has approved a novel agent, osimertinib, for T790M positive patients, considering the results obtained in the phase 3 trial AURA 3 (20).
ALK rearrangements and resistance mechanisms
Anaplastic lymphoma kinase (ALK) gene rearrangements occur in approximately 2–7% of all NSCLC cases. These activating translocations produce abnormal fusion genes and the main is EML4-ALK, which encodes a cytoplasmic chimeric protein with constitutive kinase activity that allows the activation of RAS-MERK-ERK, Janus kinase 3 (JAK3)-STAT3, and PI3K-AKT pathways. This rearrangement is more frequently found in ADC, younger patients and never or light smokers. The targeted therapy is Crizotinib, a MET, ROS1, and ALK inhibitor, that has demonstrated an initial overall response ORR of 60.8% in ALK-positive patients, with a disease control rate of 71% and a median PFS of 9.7 months. All the findings, lead the FDA to approve Crizotinib for ALK-rearrangement NSCLC (21).
This benefit is relatively short-lived and secondary to acquired resistance. Multiple secondary ALK mutations have been identified (22,23). Ceritinib, a second generation ALK inhibitor, has greater potency compared to crizotinib and has received accelerated FDA approval as the preliminary results of a phase I study demonstrated ORRs of 58% and 56% in Crizotinib naïve and resistance cases, respectively (24).
Other mutations
ROS1 rearrangements appear to occur in 1–2% of NSCLC. ROS1 is located in chromosome 6 and has a high degree of amino acid homology with ALK. Young patients, never smokers and adenocarcinoma histology, are more likely to harbor a ROS-1 rearrangement. Crizotinib treatment has been demonstrated to be efficacious with a 57% response rate and 79% disease-control rate received the FDA’s approval (25,26).
Kirsten rat sarcoma virus (KRAS) is a Ras family member of GPTases that promotes cell growth and division through Ras/raf signaling. KRAS mutations occur in 15–20% of NSCLC and are associated with smoking and adenocarcinoma histology (27,28). KRAS has been considered a non-druggable target that predicts resistance to EGFR-TKIs in NSCLC. KRAS mutations are mutually exclusive with EGFR mutations and ALK rearrangements. Therapeutic strategies currently being clinically investigated focus primarily on interfering with the signal transduction of downstream pathways, such as PI3K and MEK. The most clinical advance made is to use cytotoxic chemotherapy with a MEK inhibitor.
Other mutated gene identified in NSCLC, around 1–2% of cases, are AKT, BRAF, DDR2, FGFR, HER2 (ERBB2), MEK1, MET, NTRK1, PI3KCA, PTEN, and RET. Currently, however, no approved therapies are available to target these mechanisms (29-33).
The era of precision medicine: the shift from tissue biopsy to liquid biopsy
Understanding the molecular profile of tumor can help clinicians decide on the most appropriate treatment course, assist in therapeutic decisions and predict resistance to treatment.
The requirement for molecular testing is particularly true for patients with advanced lung cancer (70% of the cases that present lung cancer) in which the diagnosis is usually based on a tissue biopsy. Nowadays, molecular tests are done on a tissue biopsy, but tissue specimens have shown limitations, with the main ones described below: first, patient-related, this means patient discomfort, restricted or extremely risky access possibilities. Second, tissue specimens do not reflect tumor heterogeneity (intratumoral and between primary tumor and metastases, because different metastatic sites harbor distinct genomic aberrations). Third, small sample or not enough material for all molecular tests requested. Fourth, difficulty doing rebiopsies, due to safety and injury issues as well as patient reluctance. Rebiopsies are especially interesting for monitoring real-time disease and evaluation treatment responses and prediction resistance mechanisms because solid tumors exhibit an evolution over time under selection-pressure with treatment (34).
Having a complete scenario of the whole tumor based on the information obtained from small biopsies is no easy task. Given all these facts, a new approach molecular testing is needed that acts non-invasively for diagnosis, prognosis and monitoring NSCLC evolution thanks to the development of a new generation of molecular techniques. At this point, liquid biopsies seem to be the approach that best covers these requirements. More and more scientific reports point out the advantages: for diagnostics, liquid biopsy components lead to rapid biomarker assessments for molecular profiling to choose targeted agents for whom solid biopsies are impossible because of restricted or extremely risky access possibilities. Counting CTCs as a molecular marker of prognosis, after surgery, chemotherapy and radiotherapy (35). A blood sample can provide genetic landscape of all cancerous lesions (primary and metastases). Moreover, for disease monitoring, liquid biopsy is easily repeated (only a blood sample is needed), which lead to predict drug response and resistance (Table 1).
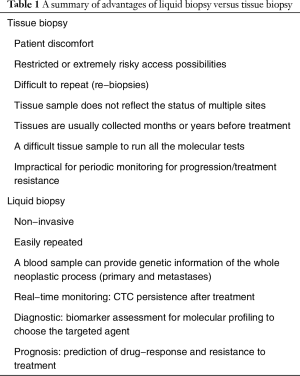
Full table
Beyond furthermore, liquid biopsies have shown the stated advantages and have begun to be introduced into clinical trials (36-38). The literature increasingly wonders which of the two most studied components [circulating tumor cells (CTCs) and cell-free DNA] is superior to the other in clinical applications (34).
In this review, we summarize the available literature on liquid biopsy in NSCLC and aim to discuss if one of the two biomarkers is superior to the other in clinical practice. This article is structured into two main sections. The first one describes the components of liquid biopsy, and the second one focuses on the clinical applications in the era of precision medicine in NSCLC.
Liquid biopsy in lung cancer
Until just a few years ago, a tissue biopsy was the gold standard for tumor diagnosis. However, as already mentioned in this review, it is a technique that cannot always be performed due to the procedure’s invasive nature and each patient’s circumstances. These reasons, together with a rising increase of cancer incidence in the population and the complexity of tumors, have meant that it is necessary to look for other studies alternatives for this disease. The so-called “liquid biopsy” could provide a solution. The possibility of investigating the molecular landscape of solid tumors through a simple peripheral blood sample may have important implications for research and patient care, which has generated considerable interest in the scientific community in the oncology field.
The term “liquid biopsy” encompasses different potential approaches for the detection of biomarkers found in any biological fluid in patients with cancer, being the peripheral blood one of the most studied. The liquid biopsy is currently an expanding field in translational cancer research and may be useful in different points of the disease, providing an early diagnosis, estimation of the risk of metastatic relapse or metastatic progression, real-time monitoring of pharmacological therapies and identification of therapeutic and resistance goals.
Within the liquid biopsy, different study elements such as CTCs, cell-free DNA, exosomes, microRNAs or platelets may be considered. They can be obtained from almost all body fluids (blood, serum, plasma, urine, pleural fluid, ascites, etc.) (39).
CTCs are a subpopulation of cancer cells that is detached from the primary tumor focus; survive in blood and can, in many cases travel to different distant organs. Circulating tumor DNA (ctDNA) fragments are released into the bloodstream by tumor cells and may contain identical genetic defects to the tumor cells that they originate from. Despite been fragmented, free DNA is stable in the bloodstream. However, free RNA molecules do not generally survive. Exceptions include cell-free microRNAs, which can be detected in the blood plasma or serum of cancer patients (40).
Relevant molecular information may also be obtained by analyzing RNA molecules present in extracellular vesicles such as exosomes or in platelets. Exosomes are nanoscale vesicles shed by most cell types that can also affect tumor biology, and their composition might promote metastasis by determining organ-specific metastatic niches.
Besides, tumor-educated blood platelets (TEPs) have been recently proposed as an alternative source of tumor-related biological information (41).
All these elements of study have contributed to provide a complete image of patients’ tumor burden, contributed more information about the tumor-related genetic profile, which allows us to understand cancer as a dynamic disease, and opened up a wide range of possible clinical applications in lung cancer (42).
However, the two liquid biopsy components used to study lung cancer that this review focuses on are CTCs and ctDNA.
CTCs: detection methods and clinical applications
CTCs are heterogeneous cell populations that circulate as either single cells or tumor cell clusters, and are assumed to contain subpopulations with metastatic potential or the ability to re-circulate back to the tumor (39).
CTCs may be detected in the blood of patients with advanced NSCLC, and may be related to these patients’ prognoses. Previous studies have shown that CTC counts are related to a poor prognosis in many metastatic cancers.
CTC detection techniques have been developed, however only a small number of CTCs can be found in the bloodstream, compared to blood cells, it is necessary to perform enrichment prior isolation (43).
Enrichment strategies for CTCs can be separated into label-dependent and label independent techniques. Among the label dependent techniques, immunomagnetic-based assays that target the EpCAM protein are the most commonly applied. Label-independent enrichment methods include size-based or density-based approaches. Negative depletion or the invasive capacity of tumor cells can also be used. A combination of different enrichment strategies is also practicable. Captured tumor cells are ready for molecular characterization by immunocytochemistry (ICC) using antibodies for tumor-specific markers or by PCR approaches that target tumor-specific mRNA or DNA sequences. Another possibility is to detect viable cells by protein secretion. Fluorescence in situ hybridization (FISH) can be used to detect tumor-specific gene aberrations (44).
However, no CTC test has yet been established in clinical practice in NSCLC, mainly owing to the lack of not only standardized detection methods, but also of reproducibility and accuracy in detecting CTCs. Notwithstanding, recent technological improvements have made it possible to isolate and quantitatively evaluate CTCs in the sampled peripheral blood of lung cancer patients (45).
The Cellsearch® system, which is the only CTC detection technique that has been approved for clinical use by the FDA (only for colon, breast and prostate cancer), enriches EpCAM-positive cells. Leukocytes may be negatively selected using leukocyte antigens such as CD45. After enrichment, the identity of the captured cells is generally verified by high-resolution images combined with immunocytofluorescence stains (45,46). This system is used, in conjunction with other clinical methods, to help monitor patients with metastatic breast, colorectal, or prostate cancer (46-48).
Detection systems can probably be criticized for only utilizing EpCAM and cytokeratin antigens in CTC isolation. Indeed, there is evidence to suggest that these antigens can be down-regulated during cellular processes that allow cancer cell invasion into the bloodstream. Thus, it would appear inadequate, or at least insufficient, to establish EpCAM as a unique and universal marker of CTCs in lung cancer (49).
An independent method for CTC detection based on size isolation of epithelial tumor cells, ISET (RareCell Diagnostics), performs a CTC enrichment step by size using a filtration device, followed by cytological characterization. This system has been used to detect lung CTC in both metastatic and non-metastatic lung cancer patients and has shown increased sensitivity in a wider range of patients compared to label-dependent methods such as CellSearch® (50). ISET technology allows higher CTC isolation rates to be achieved compared to the CellSearch (Veridex) system. Interestingly, a concordance rate of only 20% between both methods is depicted among patients in an early disease stage (51,52).
Among a variety of EpCAM-independent CTC-capture systems including size-based or density-based separation systems, a microfluidic system called a ‘CTC-chip’ has the advantage given its capability to capture specific cells with an antibody attached to microposts, which can isolate, quantify, and analyze circulating tumor cells from a blood sample. In the CTC-chip, blood flows past 78,000 EpCAM-coated microposts under controlled conditions, which optimize the capture of circulating tumor cells. An average of 67 cells per milliliter is isolated at high purity from virtually all the tested patients with metastatic cancers, including NSCLC, among others; but not from healthy controls. The prevalence and quantity of the CTCs isolated from patients with advanced cancer may thus, provide a measure of tumor response, whereas the high purity of such cells allows the repeated analysis of molecular markers. Despite the promising results reported in pilot studies, not enough additional studies have yet been reported to confirm or validate high performance (53,54).
One of the main difficulties in working with CTC in the lung cancer field is its use as a therapeutic tool to detect somatic mutations (55). However, different studies have succeeded in identifying the presence of EGFR activated mutations in CTCs isolated in EGFR-mutated patients. In addition, some authors have detected the T790M mutation in the CTCs collected from patients who progressed after TKI treatment (53). On the other hand, EGFR mutations in CTCs of NSCLC have been recently evaluated using sensitive technologies, such as Next Generation Sequencing (38). Similarly, other researchers have reported the results of ALK-specific hybridization (FISH) analysis in the CTCs of lung cancer patients (56).
Despite the improvements made in this field, CTCs are still not used in routine clinical practice; this is due to the difficulty of selecting a reliable lung CTC marker.
ctDNA: detection methods and clinical applications
Mandel and Métais first reported the presence of extracellular nucleic acids in the bloodstream in 1948. These investigators observed the presence of circulating DNA and RNA in plasma in both healthy and diseased individuals (57).
In the 1970s, high concentrations of cell-free DNA (cfDNA) in the serum of cancer patients were observed for the first time. Subsequently, in 1989, Stroun and colleagues reported that a part of the observed cfDNA in the plasma of cancer patients could come from cancer cells.
The origins and characteristics of cfDNA have been studied intermittently over the following decades, and indications have appeared that would be a potential biomarker for cancer detection (34).
ctDNA are cell free, tumor derived, short DNA fragments found in the bloodstream and comprise a small fraction of the total circulating cfDNA in plasma. cfDNA is mostly of germline origin from ruptured non-malignant cells whereas ctDNA are thought to result be from tumor cell apoptosis and necrosis with the release of fragmented DNA with a short half-life of several hours (39).
ctDNA analysis has multiple applications in lung cancer treatment, including analyzing tumor molecular heterogeneity, monitoring disease burden and prognosis, and the early detection of emerging therapy resistance (58).
The amount of ctDNA is currently, a limit to detect of genetic alterations in a liquid biopsy. Only a few thousand cfDNA copies per milliliter of plasma can be extracted, among which only a small fraction is clinically relevant. Therefore, highly sensitive and specific detection methods are required to provide a relevant diagnosis. This concern has led to the development of different detection methods that can be classified depending on whether or not it is based on specific approaches.
The targeted approaches that allow the detection of specific alterations are techniques like such real-time PCR, commonly used to detect mutations from formalin-fixed paraffin-embedded (FFPE) tumor tissues, or digital PCR (dPCR) which relies on real-time PCR, except for DNA templates being partitioned to obtain individual DNA molecules per entity, which is subsequently PCR-amplified and independently analyzed. It allows the sensitive detection of mutated ctDNA in a vast cfDNA background (59).
In the last years, a novel peptide nucleic acid (PNA)-mediated 5’ nuclease real-time polymerase chain reaction (PCR) (TaqMan) assay has been developed, which yields 78% sensitivity and 100% specificity. Using this assay, it has been assessed EGFR mutations in cfDNA isolated from baseline blood samples from patients included in the EURTAC trial and correlated EGFR mutation status with overall survival, progression free survival, and response to therapy (60).
Beads, Emulsion, Amplification and Magnetics (BEAMing) is another technique based on a first conventional PCR step, performed using primers that are specific of the targeted sequence and that contain known tag sequences. Emulsion PCR of amplicons is done in presence of tag-coupled magnetic beads that is easily purified. Through fluorescent mutant-specific probes, a flow cytometric analysis allows the detection and quantification of mutant versus wild-type alleles (61). In lung cancer samples, this technique has already demonstrated its potency to detect EGFR activating mutations and the T790M resistance mutation from plasma DNA samples (36,62).
Finally, NGS is based on the analysis of millions of short sequences from DNA molecules comparing to a reference sequence (63). In NGS we can distinguish different types as Tagged-amplicon deep sequencing (TAm-Seq), a first sequencing method that is adapted to detect rare diagnosis mutations in cfDNA (64); the Safe-Sequencing System (Safe-SeqS), proposed as a new tool to increase the sensitivity of massively parallel sequencing system instruments to identify rare variants (65); circulating single molecule amplification and re-sequencing technology (cSMART) is another strategy based on a similar approach that can also reduce the errors that occur during library preparation or in the sequencing phase (66); cancer personalized profiling by deep sequencing (CAPP-Seq) is a crucial step when designing of biotinylated “selectors” that are complementary of previously defined recurrent mutated regions. The diverse classes of mutations present in somatic samples, including single nucleotide variants, indels, rearrangements, and copy number alterations, may thus be detected depending on the designed “selectors” (67).
Another NGS technique is digital sequencing, in where each strand of a double-stranded cfDNA molecule is individually tagged, to allow custom software to compare the two complementary strands and to minimize the errors that occur during library preparation or in the sequencing phase (68). Last, but not least, bias-corrected targeted NGS is a new method for library preparations that allows minimizing off-targets and artefacts. Briefly, multifunctional adaptors that include sequences for single-primer amplification, barcodes for sample identification and tags for sequence identification are used in the tagging step (69,70).
As part of non-selective approaches, whole-exome sequencing or whole-genome sequencing could be achieved, which permit not only the detection of mutations, but also rearrangement and variation. Lung cancer is often diagnosed in an advanced disease stage, so ctDNA quantification as an early diagnosis tool for lung cancer has aroused great interest (70).
Many studies have shown that genetic variations in ctDNA reflect the mutational landscape of tumor tissue. Interestingly, although specificity among the various detection methods available comes close to 100%, sensitivity is generally weaker and might depend on the alteration type (69).
Some recently published studies, based on clinical trials with NSCLC patients, have demonstrated that bespoke assays run to detect ctDNA allow the characterization of the recurrent subclonal dynamics of patients, and identify a possible adjuvant chemotherapeutic resistance. These findings indicate that drug development guided by ctDNA platforms to identify a residual disease define the response of adjuvant treatment, and segment emerging subclones prior to clinical recurrence in NSCLC are now feasible (60,71).
To date, the evaluation of mutational profiles in CTC and cfDNA can help us to obtain prognostic and predictive information from our patients. On the other hand, the molecular analysis of CTC offers information on the immunocytochemical phenotype and cellular morphology; besides CTC analysis allows the detection of the presence of multiple mutations within the same cell and offers the opportunity to combine a genetic analysis with messenger RNA profiles (mRNA). However, the cfDNA analysis is appealing given its easy collection, and is able to carry out a plasma analysis without having to enrich and isolate a rare population of cells beforehand. In addition, ctDNA assays monitor disease burden and establish molecular profiles.
Nowadays although a ctDNA analysis plays a superior role to that of CTCs, there are still several obstacles to overcome. Perhaps the most reasonable option would be to combine these tools when making a diagnosis and following up lung cancer patients (72,73).
Currently, there are many studies in which different techniques followed to detect CTC and ctDNA are carried out in samples obtained from patients with advanced lung cancer (Table 2).
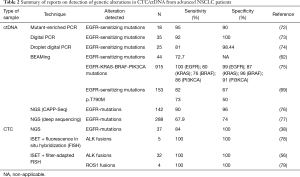
Full table
Prognostic and predictive value of CTCs and cfDNA in NSCLC
CTCs and ctDNA are useful in different lung cancer settings. They have proven valuable for early diagnosis, prognosis, prediction of treatment efficacy, monitoring responses and for the early detection of lung cancer relapse.
CTCs: currently few clinical applications
In patients at high risk of lung cancer with no clinically detectable disease, CTCs can be found and can lead to early disease detection. Surveillance using a CT-scan of CTC-positive patients in a COPD population detects lung nodules 1 to 4 years after CTC detection, which implies diagnosing early-stage lung cancer (58). The implication of these findings in patients’ outcome need to be explored in future trials.
Isolation of CTCs implies different prognoses depending on the amount of detected CTCs. More detected CTCs produces shorter OS and DFS (51,52). Absence of CTCs after chemotherapy (CT) treatment is related with better survival rates (80,81). EGFR activating mutations and the T790M resistance mutation have been detected in CTCs (37,50) as have been ALK rearrangements (56) but its value as a predictive biomarker is still to be explored.
ctDNA: today in the clinical setting
The ctDNA concentration in peripheral blood and other biological fluids increases with tumor size and disease stage (58). High sensitivity and specificity technologies are needed for early diagnosis such as digital PCR or NGS (81). Identifying plasma ctDNA in the earliest lung cancer stage patients has demonstrated the potential utility of the targeted sequencing of ctDNA in NSCLC (82).
Detecting early relapse after surgery and patients who benefit the most from adjuvant CT is possible by multi region exome sequencing (M-Seq). Recently, a prospective study has shown that ctDNA profiling can characterize the subclonal dynamics of relapsing NSCLC and has identified adjuvant chemotherapy resistance. This information leads to a new research field in which ctDNA can be used to develop new drugs, identify residual disease, define adjuvant treatment response and target emerging subclones prior to clinical recurrence in NSCLC (69). In the adjuvant setting, another prospective study has demonstrated that chromosome instability is associated with an increased risk of recurrence or death, which supports the potential value of chromosome instability as a prognostic predictor (6).
High ctDNA levels are an indicator of poor outcome in lung cancer patients, while declining ctDNA after CT treatment can be a surrogate biomarker of better prognosis (60,71,83).
However, the main steps in the predictive setting are being taken, where ctDNA is being used as a biomarker. An analysis of the EGFR L858R mutation in ctDNA has been associated with PFS and OS in the EURTAC trial (14,60). Many other assays had shown the utility of ctDNA for real-time monitoring of therapeutic responses to targeted agents as a way to abrogate the use of invasive re-biopsies (84,85).
The detection of EGFR mutations in ctDNA has been approved to select patients for the first-line treatment with Gefitinib [approved by European Medical Association (EMA) (86,87)] and Erlotinib [approved by FDA (88)].
As highlighted above before, progression to a first line TKI treatment in EGFRm patients has taken place in 50–60% of the cases by the T790M resistance mutation (16,17). The AURA trials have explored the usefulness of osimertinib, a third generation TKI, to treat the patients diagnosed with the T790M EGFR resistance mutation who progressed to a first line TKI treatment (89,90).
Currently, the only test that has been approved by the FDA or EMA to detect the T790M EGFR mutation is the cobas® EGFR Mutation test, by Roche, which is a non-digital but a real-time PCR. Nevertheless, a retrospective analysis that uses the AURA 1 and AURA 2 pooled data, has evaluated the EGFR status by comparing different ctDNA detecting technologies. The results showed a better sensitivity and specificity for the digital PCR compared to the non-digital PCR technologies (37). Based on this result, implementing digital PCR technologies into clinical practice should be explored.
Recently, some trials have shown similar response rates for those patients with positive EGFR T790M mutations in plasma to those who were tumor positive (91).
The phase 3 trial: AURA 3, has evaluated the efficacy of osimertinib compared with CT after disease progression to a TKI first line treatment in patients who were EGFR T790M positive. The trial showed a benefit for those patients treated with osimertinib over those who underwent chemotherapy. A median PFS of 10.1 months favored the experimental arm vs. 4.4 months (HR, 0.30; 95% CI: 0.23–0.41; P<0.001) in the intention to treat the population (ITT). When the results were analyzed in the population who were EGFR T790M-positive in both tumor and plasma, the median PFS benefitted the osimertinib arm with 8.2 vs. 4.2 months for the comparative arm (HR, 0.42; 95% CI: 0.29–0.61). The ORR was better with osimertinib (71% vs. 31%) (20). In line with this, a retrospective analysis of the patients included in the AURA1 and AURA2 trials has shown that the patients with NSCLC, who were positive for EFGR-T790M mutation in plasma, had equivalent outcomes to those who were positive for the mutation by tissue biopsy (37). This finding supports using plasma analysis to avoid tumor biopsies in patients who are positive for EGFR T790M in plasma. This algorithm has been approved by the main oncology agencies (5) and should become a standard of care.
Regarding the potential benefits of all these markers such as real-time monitoring of therapies, metastasis, relapse or progression, patient stratification, minimal residual disease detection and identifying better therapeutic targets, only the clinician’s and scientist’s outlook on the field, should answer whether these biomarkers are equivalent, complementary or serve in distinct purposes. Therefore, further investigation is required to clarify which biomarker is robust. In any case, the use of CTCs & ctDNA would be valuable for clinical settings in which biopsies collection is not advised or not feasible.
Conclusions
Nowadays, liquid biopsy is a milestone in the precision medicine field. The clinical application of a liquid biopsy in NSCLC is proving to be a fundamental tool in different lung cancer settings but mainly in choosing the best treatment for EGFR mutated patients and for monitoring therapy responses. In this setting, CTCs and ctDNA are the most developed technologies, but both have their own limitations.
As we have described, CTCs offer the opportunity to study whole cells, and the advantage of being able to carry out studies in vivo. Their utility in lung cancer screening has been explored, and has shown a correlation between CTC appearing in COPD patients and lung cancer early diagnosis in complementary CT-scans. Once failure treatment or disease recurrence occurs, molecular analyses of persisting CTCs may assist to select target therapies. Today CTCs are still in an early proof-of-principle stage but some advances have been made in culture CTC in vitro and expand CTC in vivo by use xenograft in lung cancer by, for example, using a 3D co-culture model, or by simulating a tumor microenvironment.
Although CTCs are proving promising results, some limitations to be addressed; e.g., the standardization of the sampling procedure to reduce pre-analytical variability, among others. On the other hand, ctDNA is winning the race from the laboratory to the clinical practice. It analysis should be chosen to study mutations, alterations in copy number, changes in DNA methylation, and also for the early detection of acquired resistance mechanisms. At this point, it is important to highlight the recent approval of the analysis that uses a liquid biopsy of the EGFR T790M status in NSCLC in the phase III trial of osimertinib in which plasma testing with an allele specific PCR assay for a ctDNA analysis showed >95% analytical sensitivity. In this setting, new digital PCR-based technology, such as droplet digital PCR or BEAMing, are offering a better analytical potential and will probably substitute real time PCR in the clinical setting.
In conclusion, the analyses of CTCs and ctDNA in NSCLC offer different analytical opportunities and, therefore, provide complementary information. Nowadays however, ctDNA can be implemented into clinical practice, while CTCs still need to undergo clinical validation.
Future clinical trials should focus on interventional studies to demonstrate the clinical utility of a liquid biopsy, where therapy decisions are based on a liquid biopsy analysis and established endpoints, e.g., time of progression or overall survival as in the phase III trial of osimertinib.
In addition to CTC and ctDNA, circulating exosomes and blood platelets might become promising candidates as novel blood-based biomarkers. These advantages in both basic and translational research will ultimately impact patient management and outcomes.
Acknowledgements
Funding: This work was supported by the RD12/0036/0025, ISCIII and grant from the Fondo Europeo de Desarrollo Regional (FEDER). E Duréndez-Sáez has a predoctoral fellowship by Asociación Española Contra el Cáncer Valencia (AECC).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global Cancer Statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Gridelli C, Rossi A, Carbone DP, et al. Non-small-cell lung cancer. Nat Rev Dis Prim 2015;1:15009. [Crossref] [PubMed]
- Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol 2013;31:992-1001. [Crossref] [PubMed]
- Goldstraw P. editor. Staging manual in thoracic oncology. FL: Editorial Rx Press, 2017:55-80.
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Barnfield PC, Ellis PM. Second-Line Treatment of Non-Small Cell Lung Cancer: New Developments for Tumours Not Harbouring Targetable Oncogenic Driver Mutations. Drugs 2016;76:1321-36. [Crossref] [PubMed]
- Meador CB, Micheel CM, Levy MA, et al. Beyond Histology: Translating Tumor Genotypes into Clinically Effective Targeted Therapies. Clin Cancer Res 2014;20:2264-75. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Rosell R, Ichinose Y, Taron M, et al. Mutations in the tyrosine kinase domain of the EGFR gene associated with gefitinib response in non-small-cell lung cancer. Lung Cancer 2005;50:25-33. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol 2013;31:3987-96. [Crossref] [PubMed]
- Lee JK, Shin J-Y, Kim S, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study. Ann Oncol 2013;24:2080-7. [Crossref] [PubMed]
- Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013;19:1389-400. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-Rearranged Non-Small-Cell Lung Cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Lin JJ, Ritterhouse LL, Ali SM, et al. ROS1 Fusions Rarely Overlap with Other Oncogenic Drivers in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:872-7. [Crossref] [PubMed]
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [Crossref] [PubMed]
- Jänne PA, Smith I, McWalter G, et al. Impact of KRAS codon subtypes from a randomised phase II trial of selumetinib plus docetaxel in KRAS mutant advanced non-small-cell lung cancer. Br J Cancer 2015;113:199-203. [Crossref] [PubMed]
- Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38-47. [Crossref] [PubMed]
- Wang L, Hu H, Pan Y, et al. PIK3CA mutations frequently coexist with EGFR/KRAS mutations in non-small cell lung cancer and suggest poor prognosis in EGFR/KRAS wildtype subgroup. PLoS One 2014;9:e88291. [Crossref] [PubMed]
- Dutt A, Ramos AH, Hammerman PS, et al. Inhibitor-Sensitive FGFR1 Amplification in Human Non-Small Cell Lung Cancer. PLoS One 2011;6:e20351. [Crossref] [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung Cancer That Harbors an HER2 Mutation: Epidemiologic Characteristics and Therapeutic Perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med 2013;19:1469-72. [Crossref] [PubMed]
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653-60. [Crossref] [PubMed]
- Calabuig-Fariñas S, Jantus-Lewintre E, Herreros-Pomares A, et al. Circulating tumor cells versus circulating tumor DNA in lung cancer—which one will win? Transl Lung Cancer Res 2016;5:466-82. [Crossref] [PubMed]
- Zhang Z, Xiao Y, Zhao J, et al. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 2016;21:519-25. [Crossref] [PubMed]
- Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015;90:509-15. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Marchetti A, Del Grammastro M, Felicioni L, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: Toward a real-time liquid biopsy for treatment. PLoS One 2014;9:e103883. [Crossref] [PubMed]
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Pantel K. Blood-Based Analysis of Circulating Cell-Free DNA and Tumor Cells for Early Cancer Detection. PLoS Med 2016;13:e1002205. [Crossref] [PubMed]
- Best MG, Sol N, Kooi I, et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015;28:666-76. [Crossref] [PubMed]
- Molina-Vila MA, Mayo-de-las-Casas C, Giménez-Capitán A, et al. Liquid Biopsy in Non-Small Cell Lung Cancer. Front Med (Lausanne) 2016;3:69. [Crossref] [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. New Engl J Med 2004;351:781-91. [Crossref] [PubMed]
- Riahi R, Gogoi P, Sepehri S, et al. A novel microchannel-based device to capture and analyze circulating tumor cells (CTCs) of breast cancer. Int J Oncol 2014;44:1870-8. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420-30. [Crossref] [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. [Crossref] [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. [Crossref] [PubMed]
- Pecot CV, Bischoff FZ, Mayer JA, et al. A novel platform for detection of CK+ and CK- CTCs. Cancer Discov 2011;1:580-6. [Crossref] [PubMed]
- Hou J-MM, Krebs M, Ward T, et al. Circulating Tumor Cells as a Window on Metastasis Biology in Lung Cancer. Am J Pathol 2011;178:989-96. [Crossref] [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [Crossref] [PubMed]
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: Comparison of the efficacy of the CellSearch AssayTM and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. [Crossref] [PubMed]
- Maheswaran S, Sequist L V, Nagrath S, et al. Detection of Mutations in EGFR in Circulating Lung-Cancer Cells. N Engl J Med 2008;359:366-77. [Crossref] [PubMed]
- Sequist L V., Nagrath S, Toner M, et al. The CTC-Chip: An Exciting New Tool to Detect Circulating Tumor Cells in Lung Cancer Patients. J Thorac Oncol 2009;4:281-3. [Crossref] [PubMed]
- Costa DB. Identification of somatic genomic alterations in circulating tumors cells: Another step forward in non-small-cell lung cancer? J Clin Oncol 2013;31:2236-9. [Crossref] [PubMed]
- Pailler E, Adam J, Barthélémy A, et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J Clin Oncol 2013;31:2273-81. [Crossref] [PubMed]
- Mandel P, Metais P. Les acides nucleiques du plasma sanguin chez l’homme. C R Seances Soc Biol Fil 1948;142:241-3. [PubMed]
- Bettegowda C, Sausen M, Leary RJ, et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci Transl Med 2014;6:224ra24. [Crossref] [PubMed]
- Vendrell JA, Mau-Them F, Béganton B, et al. Circulating Cell Free Tumor DNA Detection as a Routine Tool forLung Cancer Patient Management. Int J Mol Sci 2017;18:264. [Crossref] [PubMed]
- Karachaliou N, Mayo-de las Casas C, Queralt C, et al. Association of EGFR L858R Mutation in Circulating Free DNA With Survival in the EURTAC Trial. JAMA Oncol 2015;1:149-57. [Crossref] [PubMed]
- Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008;14:985-90. [Crossref] [PubMed]
- Taniguchi K, Uchida J, Nishino K, et al. Quantitative detection of EGFR mutations in circulating tumor DNA derived from lung adenocarcinomas. Clin Cancer Res 2011;17:7808-15. [Crossref] [PubMed]
- Murtaza M, Dawson S-J, Tsui DW, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013;497:108-12. [Crossref] [PubMed]
- Martinez P, McGranahan N, Birkbak NJ, et al. Computational optimisation of targeted DNA sequencing for cancer detection. Sci Rep 2013;3:3309. [Crossref] [PubMed]
- Kinde I, Wu J, Papadopoulos N, et al. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A 2011;108:9530-5. [Crossref] [PubMed]
- Couraud S, Vaca-Paniagua F, Villar S, et al. Noninvasive diagnosis of actionable mutations by deep sequencing of circulating free DNA in lung cancer from never-smokers: a proof-of-concept study from BioCAST/IFCT-1002. Clin Cancer Res 2014;20:4613-24. [Crossref] [PubMed]
- Newman AM, Bratman S, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 2014;20:548-54. [Crossref] [PubMed]
- Lanman RB, Mortimer SA, Zill OA, et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PLoS One 2015;10:e0140712. [Crossref] [PubMed]
- Karlovich C, Goldman JW, Sun J-M, et al. Assessment of EGFR Mutation Status in Matched Plasma and Tumor Tissue of NSCLC Patients from a Phase I Study of Rociletinib (CO-1686). Clin Cancer Res 2016;22:2386-95. [Crossref] [PubMed]
- Paweletz CP, Sacher AG, Raymond CK, et al. Bias-Corrected Targeted Next-Generation Sequencing for Rapid, Multiplexed Detection of Actionable Alterations in Cell-Free DNA from Advanced Lung Cancer Patients. Clin Cancer Res 2016;22:915-22. [Crossref] [PubMed]
- Sirera R, Bremnes RM, Cabrera A, et al. Circulating DNA is a useful prognostic factor in patients with advanced non-small cell lung cancer. J Thorac Oncol 2011;6:286-90. [Crossref] [PubMed]
- He C, Liu M, Zhou C, et al. Detection of epidermal growth factor receptor mutations in plasma by mutant-enriched PCR assay for prediction of the response to gefitinib in patients with non-small-cell lung cancer. Int J Cancer 2009;125:2393-9. [Crossref] [PubMed]
- Yung TK, Chan KC, Mok TS, et al. Single-Molecule Detection of Epidermal Growth Factor Receptor Mutations in Plasma by Microfluidics Digital PCR in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2009;15:2076-84. [Crossref] [PubMed]
- Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep 2016;6:20913. [Crossref] [PubMed]
- Janku F, Angenendt P, Tsimberidou AM, et al. Actionable mutations in plasma cell-free DNA in patients with advanced cancers referred for experimental targeted therapies. Oncotarget 2015;6:12809-21. [Crossref] [PubMed]
- Newman AM, Lovejoy AF, Klass DM, et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol 2016;34:547-55. [Crossref] [PubMed]
- Uchida J, Kato K, Kukita Y, et al. Diagnostic Accuracy of Noninvasive Genotyping of EGFR in Lung Cancer Patients by Deep Sequencing of Plasma Cell-Free DNA. Clin Chem 2015;61:1191-6. [Crossref] [PubMed]
- Ilie M, Long E, Butori C, et al. ALK-gene rearrangement: A comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol 2012;23:2907-13. [Crossref] [PubMed]
- Pailler E, Auger N, Lindsay CR, et al. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. Ann Oncol 2015;26:1408-15. [Crossref] [PubMed]
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401. [Crossref] [PubMed]
- Li J, Shi SB, Shi WL, et al. LUNX mRNA-positive cells at different time points predict prognosis in patients with surgically resected nonsmall cell lung cancer. Transl Res 2014;163:27-35. [Crossref] [PubMed]
- Chen KZ, Lou F, Yang F, et al. Circulating Tumor DNA Detection in Early-Stage Non-Small Cell Lung Cancer Patients by Targeted Sequencing. Sci Rep 2016;6:31985. [Crossref] [PubMed]
- Sozzi G, Conte D, Leon M, et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol 2003;21:3902-8. [Crossref] [PubMed]
- Marchetti A, Palma JF, Felicioni L, et al. Early Prediction of Response to Tyrosine Kinase Inhibitors by Quantification of EGFR Mutations in Plasma of NSCLC Patients. J Thorac Oncol 2015;10:1437-43. [Crossref] [PubMed]
- Marcq M, Vallée A, Bizieux A, et al. Detection of EGFR Mutations in the Plasma of Patients with Lung Adenocarcinoma for Real-Time Monitoring of Therapeutic Response to Tyrosine Kinase Inhibitors? J Thorac Oncol 2014;9:e49-50. [Crossref] [PubMed]
- Douillard JY, Ostoros G, Cobo M, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol 2014;9:1345-53. [Crossref] [PubMed]
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55-62. [Crossref] [PubMed]
- Malapelle U, Sirera R, Jantus-Lewintre E, et al. Profile of the Roche cobas(®) EGFR mutation test v2 for non-small cell lung cancer. Expert Rev Mol Diagn 2017;17:209-15. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR Inhibitor-Resistant Non-Small-Cell Lung Cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Yang J, Ramalingam SS, Jänne PA, et al. LBA2_PR: Osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol 2016;11:S152-3. [Crossref] [PubMed]
- Remon J, Caramella C, Jovelet C, et al. Osimertinib benefit in EGFR-mutant NSCLC patients with T790M-mutation detected by circulating tumour DNA. Ann Oncol 2017;28:784-90. [PubMed]


