Cost and effectiveness of lung lobectomy by video-assisted thoracic surgery for lung cancer
Introduction
Since the beginning of the 1990s, thoracoscopic surgery has acquired widespread favour with the rapid development of associated techniques and instrumentation (1-3). The American College of Chest Physicians, in its evidence-based clinical practice guidelines, suggests that “In patients with stage I non-small cell lung cancer (NSCLC) who are considered appropriate candidates for thoracoscopic anatomic lung resection (lobectomy or segmentectomy), the use of video-assisted thoracic (VATS) surgery by surgeons experienced in these techniques is an acceptable alternative to open thoracotomy” (4). Nowadays, centers consider this to be the procedure of choice for the treatment of early-stage lung cancer (5,6).
The advent of VATS, as minimally invasive surgery, has significantly altered thoracic surgical procedures (7,8) and remarkable benefits to patients such as reduced wound pain and shorter hospital stay (9,10). Furthermore, several studies reported that provides better long-term survival benefit in lung cancer patients (11,12), the leading cause of cancer-related deaths worldwide (13). However, VATS has higher equipment costs, increased operating room times, at least initially, and a learning curve for the team (14).
Here we present a cost-effectiveness study comparing lung lobectomy by VATS vs. open thoracic surgery (OPEN) in 117 patients who were diagnosed with NSCLC.
Methods
Patients
A total of 117 NSLC patients from the Department of Thoracic Surgery of the University General Hospital of Alicante suffering lung lobectomies for lung cancer treatment were included in this study. They were divided into two categories: a prospective group (n=42) that underwent VATS technique from March 2013 to January 2015; and a retrospective group of a prospectively maintained patient’s database (n=75) who underwent OPEN from September 2001 to June 2005. All patients followed standard treatment and received information on the design and purpose of the study. Institutional review board approval and participants’ informed consents were obtained.
All patients received thorough examinations including conventional blood tests, CT scan, PET-CT and head CT or magnetic resonance imaging (MRI) to exclude distant metastasis. Mediastinoscope or endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed when chest CT scan or PET indicated N2 or N3 diseases. Indications for VATS lobectomy included: no ipsilateral thoracotomy history; no evidence of severe pleural adhesions; resectable lesions ≤5 cm; no clinical sign of N2 metastases. Surgeon experience and preference were also relative indications. Preoperative discussion of each case was mandatory. All patients› clinical data were presented to evaluate the safety and efficiency at the routine meetings.
Inclusion criteria consisted of lung lobectomy surgery with curative intent and a definitive pre- or postoperative diagnosis of NSCLC not been treated with postoperative chemotherapy or radiotherapy. For OPEN group, criteria also included having a minimum of two postoperative pulmonary function tests performed correctly. Exclusion criteria were pneumonectomy or sublobar resection, patients with unresectable disease, postoperative chemo- or radiotherapy, or rapid postoperative disease progression.
Data and lung function tests
Demographic, clinical, surgical factors (incidence of complications and 30-day mortality) were evaluated.
Pulmonary function tests were carried out in the immediate preoperative period, and 1, 3 and 12 months after surgery (15) Following the American Thoracic and European Respiratory Society (ATS/ERS) 2005 international standards (14). Pulmonary function tests (PFTs), their reliability and the association with the general disease stage measured by the Brooke score. Dynamic PFTs [forced vital capacity (FVC), forced expiratory volume in one second (FEV1), diffusion capacity (DLCO) and maximal oxygen uptake (VO2max)] was measured by the single-breath method or by spirometry was performed after bronchodilator administration. DLCO, FVC, FEV1 and FEV1/FVC ratio were obtained and expressed as percentage of predicted for age, sex, and height according to the European Community for Steel and Coal prediction equations (16,17).
Preoperative staging and follow up
Conventional blood tests, thoracic CT and brain, CT or brain MRI were done to establish absence of brain metastases when neurologic symptoms occurred. A standard follow-up was followed. Additionally, during 12 months, patients were monitored for PFTs at each time point.
Perioperative mortality was defined as death within 30 days of the operation or within the same hospital admission. Survival was recorded from day of the operation until date of death or last follow-up.
Surgery methods
Thoracoscopic lobectomy, as previously reported, was performed under epidural catheter anaesthesia and general anaesthesia with dual-lumen endotracheal tubes in the absence of rib spreading (18). Postoperative chest pain was controlled by multimodal analgesia, including the provision of epidural or continuous intravenous analgesia and/or nonsteroidal anti-inflammatory drugs, which were titrated to maintain adequate pain control in order to achieve early mobilisation. The leading group made up of 4 most experienced surgeons in our department would authority the surgery and applicable approach.
Video-assisted thoracic surgery (VATS)
A 10 mm, 30° thoracoscope was introduced through the 7th intercostal space in the midaxillary line. A 5-cm access thoracotomy was usually placed in the 4th or 5th intercostal space in the anterior axillary line for upper/middle or lower lobectomy, respectively. When necessary a third opening was performed in the back of the chest, on the posterior axillary line, at the level of the 7th or 8th intercostal space. All structures to be resected were stapled with two types of linear machines: Endo-GIA Covidien, Autosuture Company Division (US Surgical Corporation, MA, USA) or ECHELON FLEX™ 60 Powered ENDOPATH® Stapler (Ethicon Endo-Surgery Inc., OH, USA).
Open thoracotomy (OPEN)
Lobectomy via open thoracotomy was performed using a posterolateral incision, of variable length depending on the case, through the 4th or 5th intercostal space. The vascular and bronchial structures were individually dissected and divided using vascular Endo-GIA 45 mm Stapler, linear 60 or 100 mm Stapler for fissures and TA 60 mm Stapler for bronchus (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA).
Histopathological examination
Pathological staging was performed according to the 7th International Staging System for Lung Cancer. Tumors were evaluated in size, location and anatomic relationships (T), the degree of lymphatic invasion node (N), and in the presence of metastatic (M) (19).
Cost calculation
The total costs were calculated at the same time point for both groups and were calculated as the sum of: (I) cost of the length of surgery time (14.33 €/minute) and stapling loads (200–300 €/shot); (II) cost of the length of time at the intensive care unit (ICU) postoperatively (1,191.3 €/day); and (III) hospitalization cost plan (323 €/day). Since the charges were recorded in books of surgery that were already destroyed 10 years ago, stapling loading costs for retrospective OPEN group were calculated using the mean of the last 10 patients on surgery as fixed value (2,104.60 €/patient).
Incremental cost-effectiveness ratio (ICER) was calculated as the cost difference between the two interventions, divided by the difference on their effect. The following issues were included in the effectiveness assessment: hospital stay for surgery, pathological stage and survival.
All the costs were summed and expressed as euros (€). Various thresholds of willingness-to-pay (WTP) to calculate the net benefit (NB) when VATS was compared to OPEN were calculated by applying the following equation in which an intervention was considered cos-effective if it resulted in a positive NB: NB = effectiveness × WTP − cost (20). Also, incremental cost and incremental effectiveness were calculated as the difference between both surgical procedures (OPEN and VATS).
WTP refers to the amount of money the payer is willing to pay for an outcome. The commonly cited WTP threshold, 50,000–100,000 USD/life year (LY), means that the payer is generally willing to pay this amount to gain a year of life and is usually considered as a threshold to decide whether an intervention is cost-effective or not (21,22). This WTP range also covers the WHO criteria (3 times gross domestic product per capita) regarding cost-effectiveness in Spain is around 101.505€ with a positive monetary net gain, so it is also cost-effective at this specific WTP level. When the incremental NB (INB) of an intervention is positive at a specific WTP level, this intervention is associated with a positive financial gain and, thus, is also cost-effective at this specific WTP level.
Statistical analysis
All variables followed a normal distribution, thus, parametric tests were used. Continuous variables are presented as means and categorical variables are expressed as absolute numbers and/or percentages. t-test for independent samples and the Pearson χ2 were used to assess variations in the study parameters between groups. A stepwise regression was use to cost with all clinical variables. Patients underwent lung tests at 1, 3 and 12 months postoperatively, and the data were compared with those gathered preoperatively. All statistical analyses were performed with the R (3.3.1) y R-Studio (0.99.892) for MAC-OSX. P<0.05 were considered to indicate significance for all parameters.
Results
Patient’s preoperative data
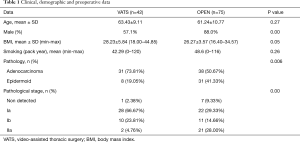
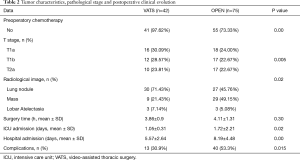
Patient’s demographic and clinical data are shown in Tables 1 and 2.
Full table
Full table
One hundred seventeen patients underwent anatomical lung resection for NSCLC, 42 of them by VATS (36%; age: 63.4±9.1 years old, 57% males) and 75 by OPEN (64%; age: 61.2±10.7 years old, 88% males). In OPEN, male frequency was significantly higher probably due to gender smoking habit (73.3%, P=0.00), less overweight (BMI 26.3±3.6 kg/m2, P=0.05), less adenocarcinoma and more epidermoid lung cancer (50.67% and 41.33%, respectively P=0.006).
Pathological tumor stage was mostly stage Ia for VATS (67%), meanwhile for OPEN was mostly stages Ia and IIa (29.3% and 28%, respectively). In OPEN group, preoperatory neoadjuvant chemotherapy was significantly higher than for VATS (OPEN: n=20, 26.7%; VATS: n=1, 2.4%, P=0.00). None of the patients received postoperatory chemotherapy. In VATS, the most frequent lobectomies were of the right upper (12/42 patients, 28.6%) and left lower lobes (11/42 patients, 26.2%). In comparison, most frequent OPEN lobectomies were of the left (28/75 patients, 37.3%) and the right upper (25/75 patients, 33.3%) lobes.
A sub analysis was done with stage II non-small cell lung (tumor present in the lung that may have spread to local lymph nodes, but has not spread further, without any preoperative significant differences between OPEN and VATS group (data not shown).
Patient’s postoperative data
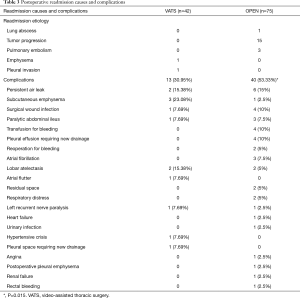
Number of complications was significantly higher in OPEN (53.3%) than VATS group (30.9%, P=0.015) mostly related to subcutaneous emphysema in VATS and for both, prolonged pulmonary air leak (Table 3). All cases recovered and no operative or 30 days after surgery deaths were registered. A total of 21 readmissions occurred along the 12 months of study (2 for VATS and 19 for OPEN, P=0.001) mostly due to tumor progression.
Full table
During the first year, in VATS group, 2 deaths due to massive pulmonary embolism and progression of neoplastic disease were recorded. In contrast, 43 patients died in OPEN group, 5 patients died in the first year, 14 in the second, 8 in the third, 7 during the fourth, 3 during the fifth, and the rest (6) at a later date. Because VATS inclusion finished in January 2015, survival at long term cannot be compared.
Lung function tests
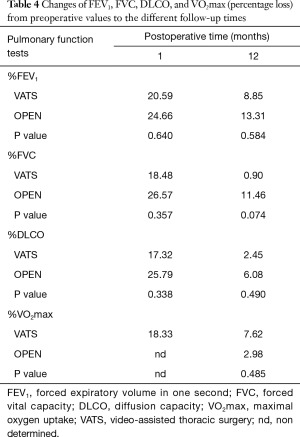
Table 4 and Figure 1 show the evolution of postoperative lung function parameters.
Full table
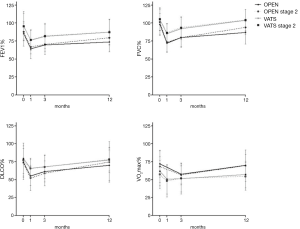
Preoperative functional lung test scores decreased right after surgery with an improving tendency during 12 months of follow-up: FEV1 (preoperative vs. 12 months in VATS vs. OPEN: 8.85% vs. 13.31%, respectively, P=0.584), FVC (0.9% vs. 11.46%, P=0.074), DLCO (2.45% vs. 6.08%, P=0.490), and VO2max (7.62% vs. 2.98%, P=0.485).
Values for most of the parameters in VATS group were higher than in OPEN, except for VO2max, which was higher in the OPEN group maybe because they were performed with a treadmill (that usually gives higher values) instead of the cycle-ergometer that was used for VATS.
The mean postoperative values presented parallel patterns, with lower values in OPEN patients. Patients from VATS group tended to recover FEV1 and FVC quicker than patients from OPEN group. Similar PFT results were obtained when only stage II NSCLC patients were analyzed.
Cost-effectiveness analysis
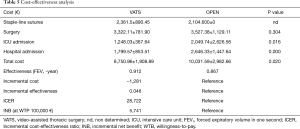
Cost-effectiveness analysis data is presented in Table 5. There were statistically significant differences between the two groups in the costs of surgical equipment, number of supplies, days of hospitalization and days of ICU (Table 5). Total cost for VATS was significantly lower than for OPEN (8,751±1,909 vs. 10,032±2,983€, respectively, P=0.02). This difference was mostly related to the cost of total hospitalization days. For the entire follow-up period, no significant differences in the survival rate were found between VATS and OPEN (data not shown).
Full table
FEV1 within one year after surgery was lower for VATS vs. OPEN (0.9115 vs. 0.8669 L, respectively). Incremental cost was −1,281€ (VATS − OPEN cost = 8,751€ − 10,032€) and incremental effectiveness 0.0446(=0.9115−0.8669 L) FEV1, the NB if WTP equals €100,000/LY would be positive 3,179€ [=0.0446×100,000−(−1,281)]. The ICER when VATS was compared to OPEN was −28,722€ (=−1,281/0.0446 €/FEV1).
Thus, VATS is potentially more cost-effective than OPEN at short-term (1 year) within the common WTP levels from a payer’s perspective since VATS estimated ICER (−28,722 €/LY) was below the WHO common criteria (100,000 €/LY).
Discussion
As it is well known, VATS lobectomy has been accepted as one of the surgical therapeutic methods for lung cancer resection by the National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology mainly because presents a tendency of less damage to respiratory function, in the same way in early tumor stages, less complications being the cost of this technique lower. The results of the present study are in line with previous preliminary estimates (14,23,24).
Jiao et al. set at 6 months the time-point to evaluate the permanent lung functional loss after VATS lobectomy. Usually, there is no further significant improvement in pulmonary function tests in the follow-up period (25). However, in our data, although must be confirmed, there was a tendency for lung function recovery at 12 months after VATS lobectomy. Meanwhile, in accordance with previous studies of Bolliger et al. (26) and Nezu et al. (27), we found that OPEN leaded to a 10% permanent loss in pulmonary function. Several reports suggested that lobectomy patients suffered a significant reduction (15%) of functional reserve at 6 months postoperatively, with almost equal deterioration between lung function and exercise capacity (28,29). Also, for many patients, the risk of impaired quality-of-life after surgery is an important consideration. Several reports suggested that pulmonary resection has a negative influence on the quality-of-life with a subsequent partial recovery over time (30-32).
It is believed that metastases occur most frequently during the perioperative period, when injury and repair are more prominent and stress can elaborate various humoral substances that potentiate the growth of carcinomas. Thus, Lewis et al. (33) proposed that VATS minimal invasive surgical procedures performed with small incisions could account for improved long-term survival. Furthermore, in VATS, fewer malignant cells would be disseminated and passed into blood vessels or lymphatics, which could occur from the extensive mechanical stress through palpation and compression common to the OPEN technique (33,34).
Moreover, VATS would potentially avoid complications, eliminate disruptions from anaesthetic induction, and shorten hospital stay, providing a more cost-effective approach (35). In the study of Paul et al. (12), 73.8% of patients who underwent VATS presented no complications, while 65.3% of patients that underwent OPEN had no complications. They also found that patients that underwent VATS had a lower incidence of arrhythmias, reintubation, blood transfusion, shorter hospital stay, and chest tube duration (12). In addition, to these early functional advantages, VATS lobectomy has been shown to have similar long-term outcomes compared to OPEN (36,37).
Swanson et al. compared hospital costs and perioperative outcomes for VATS (n=1,054) and OPEN (n=2907), in the United States using the premier prospective length of stay was 7.83 days for OPEN vs. 6.15 days for VATS. As in our study, surgery duration was shorter for VATS vs. OPEN procedures (3.75 vs. 4.09 hours). Also, the risk of adverse events was significantly lower in VATS group (P=0.019) and hospital costs were higher for OPEN vs. VATS [$21,016 vs. $20,316 (P=0.027)]. Higher costs for OPEN surgery were confirmed in several studies (38,39). In addition, our results show that VATS is potentially more cost-effective than OPEN within the common WTP levels.
However, our study presented some limitations. The main limitations were: (I) the different period of study; (II) different diagnostic and staging methods; (II) different surgeons performing the procedures. In addition, there is always concern in potential unobserved confounding bias especially being VATS prospective and OPEN retrospective, taking into account the gender differences. Secondly, although the long term outcome of early stage NSCLC was quite good, the duration of our study (1 year) might not be long enough to fully capture the cost-effectiveness, and, probably longer follow-up might make VATS more favourable given the slightly improved survival and similar cost at the end of our follow-up period. Furthermore, the number of patients in our study is relatively low and further studies with larger groups should be performed. In addition, similar tumor stages should be compared in order to estimate survival and cost-effectiveness and OPEN costs were calculated from the last 10 surgeries. Another possible bias of the VATS technique could be that smaller tumors without affecting the pulmonary hilum or nearby structures will be operated.
We provided evidence that VATS is a safe and feasible technique and, when compared to OPEN, it was potentially more cost-effective at short term (1 year) within the common WTP levels from payer’s perspective in Spain. Further studies would be helpful to see the long-term cost-effectiveness results and whether the same results could be obtained in other health care systems.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lewis RJ, Caccavale RJ, Sisler GE, et al. One hundred consecutive patients undergoing video-assisted thoracic operations. Ann Thorac Surg 1992;54:421-6. [Crossref] [PubMed]
- Kirby TJ, Rice TW. Thoracoscopic lobectomy. Ann Thorac Surg 1993;56:784-6. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Kaseda S, Aoki T, Hangai N. Video-assisted thoracic surgery (VATS) lobectomy: the Japanese experience. Semin Thorac Cardiovasc Surg 1998;10:300-4. [Crossref] [PubMed]
- Walker WS. Video-assisted thoracic surgery (VATS) lobectomy: the Edinburgh experience. Semin Thorac Cardiovasc Surg 1998;10:291-9. [Crossref] [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007;12:20-37. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Brusasco V, Crapo R, Viegi G, et al. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J 2005;26:1-2. [Crossref] [PubMed]
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5-40. [Crossref] [PubMed]
- Cotes JE, Chinn DJ, Quanjer PH, et al. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:41-52. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Fang HY, Hsiao FY, Huang HC, et al. Cost and effectiveness of video-assisted thoracoscopic surgery for clinical stage I non-small cell lung cancer: a population-based analysis. J Thorac Dis 2014;6:1690-6. [PubMed]
- Konski A. Economic analysis of health care interventions. Semin Radiat Oncol 2008;18:168-74. [Crossref] [PubMed]
- Shih YC, Halpern MT. Economic evaluations of medical care interventions for cancer patients: how, why, and what does it mean? CA Cancer J Clin 2008;58:231-44. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:234S-42S.
- Jiao W, Zhao Y, Wang M, et al. A retrospective study of diaphragmatic motion, pulmonary function, and quality-of-life following video-assisted thoracoscopic lobectomy in patients with nonsmall cell lung cancer. Indian J Cancer 2015;51 Suppl 2:e45-8. [Crossref] [PubMed]
- Bolliger CT, Jordan P, Soler M, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996;9:415-21. [Crossref] [PubMed]
- Nezu K, Kushibe K, Tojo T, et al. Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 1998;113:1511-6. [Crossref] [PubMed]
- Win T, Groves AM, Ritchie AJ, et al. The effect of lung resection on pulmonary function and exercise capacity in lung cancer patients. Respir Care 2007;52:720-6. [PubMed]
- Nagamatsu Y, Iwasaki Y, Hayashida R, et al. Factors related to an early restoration of exercise capacity after major lung resection. Surg Today 2011;41:1228-33. [Crossref] [PubMed]
- Brunelli A, Pompili C, Koller M. Changes in quality of life after pulmonary resection. Thorac Surg Clin. 2012;22:471-85. [Crossref] [PubMed]
- Pompili C, Brunelli A, Xiume F, et al. Predictors of postoperative decline in quality of life after major lung resections. Eur J Cardiothorac Surg 2011;39:732-7. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5. [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Does VATS favor seeding of carcinoma of the lung more than a conventional operation? Int Surg 1997;82:127-30. [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. 85 consecutive VATS non-rib spread simultaneously stapled lobectomies for malignancy. Zentralbl Chir 1998;123:501-5. [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- D'Amico TA. Long-term outcomes of thoracoscopic lobectomy. Thorac Surg Clin 2008;18:259-62. [Crossref] [PubMed]
- Yamamoto K, Ohsumi A, Kojima F, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg 2010;89:353-9. [Crossref] [PubMed]
- David G, Gunnarsson CL, Moore M, et al. Surgeons' volume-outcome relationship for lobectomies and wedge resections for cancer using video-assisted thoracoscopic techniques. Minim Invasive Surg 2012;2012:760292. [Crossref] [PubMed]
- Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc 2011;25:1054-61. [Crossref] [PubMed]


