Adverse events with bioresorbable vascular scaffolds in routine percutaneous coronary interventions: “coup de théâtre” or unfinished play?
Bioresorbable scaffolds are percutaneous coronary prostheses designed to ensure transient support of the dilated vessel and dissolution into inert breakdown products overtime (1). By leaving the coronary artery without a permanent metallic implant, bioresorbable scaffolds have been developed to absolve three main commitments: improve long-term vessel healing and remodelling, restore vasomotor function of the treated segment, and reduce the burden of angina symptoms in comparison with conventional drug-eluting stent (DES) platforms.
The encouraging short-term data from four industry-sponsored randomized controlled trials granted the everolimus-eluting bioresorbable vascular scaffold (BVS/Absorb, Abbott Vascular, Santa Clara, CA, USA) the approval from regulatory agencies for clinical use in Europe, USA, China and Japan (2). In these pivotal trials, patients with moderately complex obstructive coronary artery disease (CAD) treated with either BVS or everolimus-eluting metallic stent (EES) showed broadly comparable clinical outcomes at 12 months. However, a meta-analysis (3) and a large registry of individuals with relatively more complex CAD (4) displayed a thrombotic risk at 6- to 12-month follow-up with BVS about twice as high in comparison with the benchmark metallic EES. More disappointingly, recent follow-up data beyond 1 year from those trials (5,6) originally designed to support the regulatory approval of BVS in Europe and Japan failed to demonstrate either physiological or clinical advantages with BVS as compared to EES. At the opposite, BVS was associated with a risk of failure, which accrued with the time.
In line with these considerations, the results of the Amsterdam Investigator-initiateD Absorb strategy all-comers (AIDA) trial published in the New England Journal of Medicine in 2017 have to be highlighted (7). The AIDA trial was a single blind, multicentre, investigator-initiated, non-inferiority, randomized clinical trial, which included 1,845 unselected CAD patients with 2,446 lesions assigned to either BVS or EES therapy. The primary endpoint was target vessel failure (TVF) the composite of cardiac death, target vessel related myocardial infarction (TV-MI) or target vessel revascularization at 2 years. Device thrombosis at 2 years was a main secondary endpoint. Among those enrolled, 54.1% of patients presented an acute coronary syndrome at admission (including one-fourth of participants with ST-segment elevation MI) and 52.8% of lesions treated had a complex morphology. In this report, the authors provided descriptive outcomes data released after a median follow-up duration of 2 years since the data and safety monitoring board recommended early reporting of results owing to safety concerns. The main findings of AIDA trial were as follows: at 2-year follow-up (I) BVS was associated with a risk of TVF comparable to that of EES; (II) however, BVS had an approximately 3.5 times higher risk of thrombosis and a higher risk of TV-MI as compared to EES; (III) notably, nearly one-third of BVS thromboses occurred in the period beyond 1 year after implantation. These results deserve an in-depth discussion.
First, although the acronym of the trial by Wykrzykowska et al. recalls the name of the Nubian princess protagonist of the homonymous play by Giuseppe Verdi, the results of the AIDA trial do not represent a “coup de théâtre”. Indeed, 10 days before the New England Journal of Medicine posted online the results of this trial, the 2-year data from the ABSORB III trial presented during the 2017 American College of Cardiology Congress reported a higher risk for device failure and thrombosis associated with BVS as compared to EES (8). Similarly, the majority of recently published meta-analyses (1,9-18) investigating the clinical outcomes beyond 1 year of CAD patients treated with either BVS or EES display a consistent higher risk of failure and/or thrombosis with fully-bioresorbable scaffolds regardless of the availability of the AIDA trial for pooled risk estimates (Table 1).
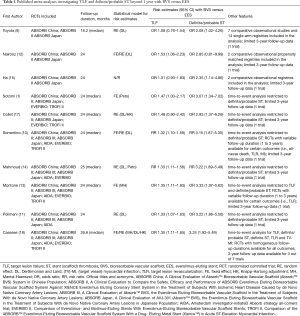
Full table
Second, although some enthusiastic proponents of BVS technology continue to argue that a suboptimal implantation technique accounts for the observed unfavourable results (19), there is no evidence from randomized trials or meta-analyses of individual data in support of this claim. Investigators of AIDA trial were experienced in BVS technology at time of study inception and conduction, as indicated from the high proportion (96.9%) of patients in the BVS group in which at least one assigned study device was implanted, the few number of devices implemented in vessels <2.25 mm in diameter and the proportions of pre- and post-dilation in the group of patients treated with BVS (96.9% and 74%, respectively). These percentages were significantly higher than those of the group of patients treated with EES and among the highest reported within randomized trials studying this technology (18). Notably in this trial, BVS failure occurred regardless of pre- and post-dilation and of the use of a specific protocol dedicated to BVS implantation (pre-dilation, appropriate vessel sizing, and high-pressure post-dilation) suggesting that device failure may not only depend on technical issues (20). In addition, although the adoption of BVS implantation protocols targeted at improving acute mechanical results may hypothetically impact on short-term outcomes (21), whether such protocols can modify rates of late thrombotic events remains to be demonstrated.
Third, the accrual of thrombotic events after the discontinuation of dual antiplatelet therapy (DAPT) observed in this as well as in other randomized trials of BVS versus metallic DES, has prompted the AIDA investigators (22) and certain professional associations (23) to recommend the prolongation of DAPT up to 36 months or even the re-introduction of DAPT in all patients treated with BVS if not contra-indicated. Moreover, some experts have suggested intensifying platelet inhibition with more potent P2Y12-receptor antagonists in patients treated with BVS at low risk of bleeding (24). However, these recommendations are based on experts’ consensus rather than on a solid evidence. In this regard, despite the ABSORB II trial did not report late thrombotic events after BVS implantation in patients that never interrupted DAPT out to 3 years (6), a recent meta-analysis found a higher thrombotic risk with BVS both at 12- and 24-month follow-up, irrespectively of the proportions of patients on DAPT at each timepoint (18). Moreover, the well-known increased risk of bleeding associated with a prolonged DAPT (25) may nullify the potential long-term benefits of fully-bioresorbable platforms, which are expected some years after implantation.
In consideration of these indicators of concern, the Food and Drug Administration warned all practitioners in the USA against the higher risk of adverse events associated with BVS, recommending a careful patient selection and the implementation of implantation protocols specific to this technology. Concomitantly, after advising in conjunction with the European Regulatory Agency all physicians regarding the worrisome mid-term outcomes of BVS, the manufacturer has discontinued the normal commercial sale of BVS in Europe and restricted its usage to centres participating in carefully monitored registries. Notwithstanding this, the fact that the performance of current BVS does not reflect initial enthusiastic expectations should not jeopardize further investigations in this field, leaving the technology of fully-bioresorbable scaffolds as an “unfinished play”. The knowledge of intrinsic limitations of current devices should encourage a continuous iteration towards new-generation fully-bioresorbable scaffolds, which are in advanced phases of development (1). The hypothesis that refinements of implantation protocols and ancillary therapies might reduce adverse events in patients treated with current BVS generation is under investigation in ongoing large-scale randomized trials (NCT02173379, NCT02486068). In the meantime, long-term follow-up data (>5 to 10 years) of completed studies and future comparative studies of new-generation fully-bioresorbable platforms remain fundamental to disclose possible advantages of this technology in comparison with contemporary high-performance metallic DES. Until further data will be available, current benchmark metallic DES should be further regarded as standard comparator for studies investigating the relative safety and efficacy of different percutaneous technologies for patients undergoing revascularization because of obstructive CAD.
Acknowledgements
None.
Footnote
Conflicts of Interest: A Kastrati has submitted patents in relation to DES technologies. The other authors have no conflicts of interest to declare.
References
- Sotomi Y, Onuma Y, Collet C, et al. Bioresorbable Scaffold: The Emerging Reality and Future Directions. Circ Res 2017;120:1341-52. [Crossref] [PubMed]
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016;387:1277-89. [Crossref] [PubMed]
- Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2016;387:537-44. [Crossref] [PubMed]
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015;10:1144-53. [Crossref] [PubMed]
- Onuma Y, Sotomi Y, Shiomi H, et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention 2016;12:1090-101. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med 2017;376:2319-28. [Crossref] [PubMed]
- Ellis SG, Kereiakes DJ, Stone GW, for the ABSORB III Investigators. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results. American College of Cardiology Congress; 2017 March 19; Washington DC, USA.
- Toyota T, Morimoto T, Shiomi H, et al. Very Late Scaffold Thrombosis of Bioresorbable Vascular Scaffold: Systematic Review and a Meta-Analysis. JACC Cardiovasc Interv 2017;10:27-37. [Crossref] [PubMed]
- Sorrentino S, Giustino G, Mehran R, et al. Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents. J Am Coll Cardiol 2017;69:3055-66. [Crossref] [PubMed]
- Polimeni A, Anadol R, Münzel T, et al. Long-term outcome of bioresorbable vascular scaffolds for the treatment of coronary artery disease: a meta-analysis of RCTs. BMC Cardiovasc Disord 2017;17:147. [Crossref] [PubMed]
- Nairooz R, Saad M, Sardar P, et al. Two-year outcomes of bioresorbable vascular scaffold versus drug-eluting stents in coronary artery disease: a meta-analysis. Heart 2017;103:1096-103. [Crossref] [PubMed]
- Montone RA, Niccoli G, De Marco F, et al. Temporal Trends in Adverse Events After Everolimus-Eluting Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent Implantation: A Meta-Analysis of Randomized Controlled Trials. Circulation 2017;135:2145-54. [Crossref] [PubMed]
- Mahmoud AN, Barakat AF, Elgendy AY, et al. Long-Term Efficacy and Safety of Everolimus-Eluting Bioresorbable Vascular Scaffolds Versus Everolimus-Eluting Metallic Stents: A Meta-Analysis of Randomized Trials. Circ Cardiovasc Interv 2017;10. [Crossref] [PubMed]
- Ha FJ, Nerlekar N, Cameron JD, et al. Midterm Safety and Efficacy of ABSORB Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent: An Updated Meta-Analysis. JACC Cardiovasc Interv 2017;10:308-10. [Crossref] [PubMed]
- Elias J, van Dongen IM, Kraak RP, et al. Mid-term and long-term safety and efficacy of bioresorbable vascular scaffolds versus metallic everolimus-eluting stents in coronary artery disease: A weighted meta-analysis of seven randomised controlled trials including 5577 patients. Neth Heart J 2017;25:429-38. [Crossref] [PubMed]
- Collet C, Asano T, Miyazaki Y, et al. Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Cassese S, Robert BA, Jüni P, et al. Mid-term clinical outcomes with everolimus-eluting bioresorbable scaffolds versus everolimus-eluting metallic stents for percutaneous coronary interventions: a meta-analysis of randomized trials. EuroIntervention 2017. [Epub ahead of print]. [PubMed]
- Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: development and internal validation of the PSP score. EuroIntervention 2017;12:2110-7. [Crossref] [PubMed]
- Wykrzykowska JJ, Kraak RP, Tijssen JG. Amsterdam Investigator-initiateD Absorb Strategy All-comers Trial (AIDA). EuroPCR Congress; 2017 May 19; Paris, France.
- Puricel S, Cuculi F, Weissner M, et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol 2016;67:921-31. [Crossref] [PubMed]
- Henriques JP, Elias J. The first generation ABSORB BVS scaffold; to be or not to be? Neth Heart J 2017;25:416-8. [Crossref] [PubMed]
- Everaert B, Wykrzykowska JJ, Koolen J, et al. Recommendations for the use of bioresorbable vascular scaffolds in percutaneous coronary interventions: 2017 revision. Neth Heart J 2017;25:419-28. [Crossref] [PubMed]
- Capodanno D, Angiolillo DJ. Antiplatelet Therapy After Implantation of Bioresorbable Vascular Scaffolds: A Review of the Published Data, Practical Recommendations, and Future Directions. JACC Cardiovasc Interv 2017;10:425-37. [Crossref] [PubMed]
- Cassese S, Byrne RA, Ndrepepa G, et al. Prolonged dual antiplatelet therapy after drug-eluting stenting: meta-analysis of randomized trials. Clin Res Cardiol 2015;104:887-901. [Crossref] [PubMed]


