Optical coherence tomography guidance during bioresorbable vascular scaffold implantation
In the last decades angiography has been the keystone to assess coronary anatomy, leading to a rapid development of percutaneous revascularisation techniques. Despite the widespread dissemination and high reproducibility, angiography provides a limited analysis of arterial lumen profile without the possibility to disclose vessel wall characteristics and composition of coronary plaques. Intracoronary imaging techniques have been developed to overcome these limitations. Intravascular ultrasound (IVUS) was the first technique introduced in interventional cardiology in the early 90ies (1), followed more than a decade after by optical coherence tomography (OCT). OCT is a light-based technology that similar to IVUS provides information about intravascular anatomy that far exceeds the level of detail obtained from conventional angiography (2). The use of near-infrared light rather than ultrasound reflectance allows OCT to have greater resolution at the price of lower penetration power. Moreover, near-infrared light is scattered by red blood cells, and therefore OCT use for guidance of intervention is limited by the need of prolonged crystalloid infusion during imaging (3,4). In so forth, the penetrance of OCT in daily clinical practice was limited and the technology was mainly employed as a research tool to investigate plaque morphology and strut endothelialisation (5). The frequency domain OCT system (FD-OCT) has the advantage of a more rapid image acquisition due to the fast-scanning laser systems minimising the contrast use and increasing imaging speed while delivering an improved image quality than with the earlier time domain systems (TD-OCT) (6). This allows multiple acquisitions of the entire vascular segment of interest with an amount of contrast only slightly higher than the amount required for the control angiogram (7). Another potential advantage of the FD-OCT over the old TD-OCT is that imaging acquisition does not require arterial balloon occlusion during the pullback, reducing the risk of ischemia and vascular injuries (8). However, despite the safety and high-resolution power, the use of FD-OCT during PCI is less than 5% worldwide and its real role during daily practice still have to be defined. Surely, a potential field of application of OCT is for guidance of bioresorbable vascular scaffold (BRS) implantation.
BRS represent a revolutionary concept in interventional cardiology. This technology has the potential to induce a true anatomical and functional “vascular restoration” after coronary revascularization, with the scaffold losing mechanical integrity after 6–12 months and completely reabsorbed in 3–5 years (9,10). After initial enthusiasm, justified by the positive results reported in initial small studies and by the ABSORB III randomized trial that showed a non-inferiority for target lesion failure (TLF) at 1 year between Absorb BVS (Abbot Vascular, Santa Clara, California, USA) and the Xience everolimus eluting stent (EES) (11,12), the AIDA trial and the three years follow up of the ABSORB II trial reported not trivial rates of scaffold thrombosis (ScT), requiring further investigations (13,14). These negative findings affected so much the credibility of bioresorbable therapy that the ABSORB BVS has been recently retired unless if used in controlled study protocols. Surely, the uncorrected patients and plaques selection together with suboptimal scaffold implantation might be partially related to the BRS negative results.
One of the potential pitfalls of bioresorbable scaffold is the low radial strength. While new generation metallic drug eluting stents (DES) presents a high radial strength able to counteract plaque recoil, the BRS structure is further weakened during the reabsorption process. Moreover, the presence of calcium or fibrosis that impairs distensibility of the vessel wall behind the scaffold might represent major limitations to an optimal scaffold opening. In so forth, tissue characterization and lesion preparation before BRS implantation is one of the crucial step for optimal BRS response.
OCT can accurately assess plaque characteristics such as calcifications, fibrous and lipid-rich plaque components, as well as the presence of dense macrophage infiltration, neovascularization and mural/luminal thrombi (15-19). Unfortunately, because of its limited tissue penetration, OCT is not suitable to properly visualise the external elastic membrane (EEM) in heavily diseased segments so that IVUS remains the gold standard to study vessel remodelling (19,20).
Once correct plaques characterization has been performed, the next step for optimal BRS response sees a proper lesion preparation followed by optimal scaffold apposition (Figure 1) (21-23). In fact the mechanical properties of BRS substantially differ from those of metal stents and the relatively less radial strength may results in insufficient scaffold expansion that might not be corrected by an aggressive post-dilatation because of the awareness that it might results in scaffold fracture. The lack of shadowing observed beyond polymer struts makes OCT the optimal imaging technique to optimize BRS implantation and identify eventually scaffolds failures such as malapposition, edge dissection, tissue protrusion, thrombus and fractures (24).
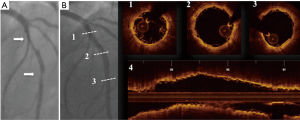
Role of OCT guidance for BRS in clinical trial and registries: apposition, coverage and reabsorption
OCT has been largely applied in dedicated cohorts of patients from major clinical BRS-trials for the assessment of acute post-procedural and long-term outcome (Table 1).
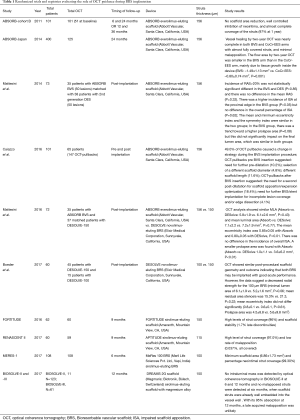
Full table
The ABSORB trial has provided the first description of the OCT appearance of the scaffolds. In the ABSORB cohort B study, enrolling 101 patients treated with the ABSORB everolimus-eluting scaffold (Abbott Vascular, Santa Clara, California), OCT was performed in 2 groups of patients, respectively at 6 and 24 months (B1, n=45) and 12 and 36 months (B2, n=56). Baseline imaging was optionally performed in 51 patients. The results showed the maintenance of the scaffold area at distance, with a slight decrease in luminal area, as a consequence on neointimal proliferation inside the BRS. In fact, 97% of the struts were covered at 1 year, with the scaffold displaying initial signs or resorption, but still largely visible (25). In addition, the comparison of post-procedural and late imaging allowed the discrimination between the acute scaffold disruption, which has been associated to an increased risk of TLF and thrombosis, and late discontinuities, representing the natural consequence of the reabsorption process (26).
In the subsequent ABSORB Japan trial, 400 patients were randomized to receive either the ABSORB BVS or cobalt-chromium EES. One-hundred twenty-five patients were randomly assigned to the OCT cohort, undergoing an imaging assessment at baseline and 2 years of follow-up. The OCT findings showed a complete vascular healing at 2 years, almost full struts coverage and minimal scaffold malapposition both in BRS and DES groups. Larger tissue growth was observed inside the BRS, resulting in a smaller flow area. The authors reported a 1.6% of very late ScT rate certainly superior to the <1% reported with newer generations of DES. OCT imaging of this subgroup of patients showed struts malapposition and discontinuities, but whether this was a late acquired “physiological” phenomenon or an acute post-implantation rupture could not be discriminated for the lack of immediate post-implantation data because OCT evaluation was performed only after the event (27).
In another study our group showed that an extensive use of OCT for guidance of BRS implantations allowed achieving similar acute performance than second generation metallic DES even during treatment of complex coronary lesions (28). We compared fifty complex coronary lesions (all type ACC/AHA B2-C) treated with BRS under OCT guidance matched to an equal number of lesions treated with second generation DES. We found a similar incidence of residual area stenosis (RAS) and overall percentage of incomplete stent apposition (ISA) between the DES-group and BRS-group. Mean and minimal lumen area were similar in the two groups with also a similar mean and minimum eccentricity as well as symmetry index between the two groups. In the BRS group, there was a trend toward a higher prolapse area but this did not significantly impact on the final lumen area. These results however required a higher balloon diameter/mean reference vessel diameter ratio for predilatation in the BRS group with significantly higher pre and post dilatation inflation pressure together with a more extensive use of NC balloons for lesion preparation in the BRS group. Despite this, our data suggest that a satisfactory scaffold expansion can be achieved also in complex coronary lesions, at least when appropriate lesion preparation and BRS deployment under OCT guidance is performed. Moreover, in a small subgroup of 22 calcified lesions we also showed the safety and feasibility of super high-pressure dilatation (max post dilatation pressure of 28±3 ATM) after BRS deployment without reporting any scaffold fractures (29).
The importance of OCT examination during BRS implantation is also highlighted by another study (30). In this retrospective analysis of more than 200 consecutive BRS implanted in 101 patients, we found that almost half of the OCT examinations led to a change in strategy before and/or after scaffolds implantation. When used before, OCT images suggested additional lesions preparation and allowed fine-tuning of the length and size of BRSs used. When used as a final control, OCT-pullback led to further post-dilatation in almost one third of the cases despite the aggressive systematic angiography-guided optimization technique used in the study. Interestingly, we also found that lesions treated with 2.5-mm overlapping scaffolds, considered at a higher degree of complexity, required more than one OCT pullback in a higher proportion of cases compared with the other lesions; not a trivial finding since the vast majority of ScT reported generally affect BRS improperly used during treatment of small coronary vessels.
Similar results, as compared to the ABSORB BVS, have also been achieved with the DESOLVE novolimus-eluting BRS (Elixir Medical Corporation, Sunnyvale, California, USA). The authors compared the OCT findings from a cohort of patients treated with the ABSORB (n=35) and 37 matched patients receiving the DESOLVE-150 scaffold. There was no difference in minimal and mean lumen area and in the incidence of struts malapposition between the two scaffolds. However, the DESOLVE-150 showed a non-significantly higher tissue prolapse and asymmetric expansion, potentially conditioned by the slightly inferior thickness of the struts as compared with the ABSORB BVS (150 vs. 156 µm). Nevertheless, differences in the characteristics of the treated lesions could also have influenced the results of the study (31).
Indeed, the relevance of the thickness of the BRS struts has emerged after the demonstration that large scaffold platforms present limits in deliverability and favour the disruption of the laminar flow, with increase in thrombogenicity. The DESOLVE-100 µm (Elixir Medical Corporation, Sunnyvale, California, USA), in fact, has been implanted in 15 patients and compared with the results of the larger DESOLVE-150 (n=45 patients). OCT showed a good mechanical acute performance of the thinner platform, with lower rates of edge dissections. However, higher rates of scaffold fractures and reduced radial force emerged with the 100µm platform, despite larger experiences are certainly needed to draw more definite conclusions (32).
A similar reduction in the thickness of the struts has been observed in the Amaranth research program (Mountain View, CA, USA), although their BRSs still represent investigational products not available on the market. The first FORTITUDE—150 µm sirolimus-eluting scaffold showed 96% struts coverage and scaffold stability at 9-months OCT examinations. The subsequent APTITUDE—115 µm sirolimus-eluting scaffold, in the RENASCENT II study, confirmed at 9-months OCT good struts coverage (97%), with a low rate of malapposition (0.037% of struts, all re-endothelized), whereas the MAGNITUDE—100 µm sirolimus-eluting scaffold is currently evaluated in the ongoing RENASCENT III study.
The MeRes 100 BVS (Meril Life Sciences Pvt. Ltd., Vapi, India) also is an analogous low-profile, sirolimus-eluting BRS, that has been implanted in 166 lesions (108 patients) in the MeRes-1 first-in-man study. The OCT re-assessment showed an early 6-months high rate of neointimal coverage (99.3%), potentially providing an explanation for the good clinical performance (low rate of MACE, 0.93%, with no ScT) at 1-year clinical follow-up (33).
Finally, a different scaffold design, has been developed for the DREAMS 2G (Magmaris; Biotronik, Bülach, Switzerland), a sirolimus-eluting scaffold built in a magnesium alloy. In the BIOSOLVE II and III program, OCT was performed on volunteer basis at 6, 12 and 36 months. No intraluminal mass was detected by OCT in BIOSOLVE-II at 6 and 12 months and no malapposed struts were detected at 6-months, when scaffold struts were already well embedded into the vessel wall, with 95% of absorption at 12-months. Thanks to its laser-polished surface the DREAMS 2G present a smooth surface, and strut cross-sections are rectangular with rounded edges, which may facilitate embedding into the vessel wall (34). Nevertheless, a dedicated Magmaris-OCT study is currently ongoing and will enrol 60 consecutive patients undergoing Magmaris scaffold implantation. The primary end point will be the percentage of uncovered scaffold struts assessed by OCT at the prespecified follow-up up to 12 months (35).
Conclusions
BRS represent a revolutionary concept in interventional cardiology with their unique potentiality to induce a true anatomical and functional “vascular restoration” after coronary revascularization. However, the mechanical properties of these polymer-based scaffolds highly differ from the metallic stents used in our daily practice. The importance of correct patients selection as well as technical aspects during BRS implantation procedures has been highlighted in several studies suggesting that the high ScT reported might be related to the underutilization of intracoronary imaging guidance during BRS implantation. OCT might represent the optimal imaging technique to optimize BRS implantation and identify eventually scaffolds failures.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yock PG, Fitzgerald PJ, Linker DT, et al. Intravascular ultrasound guidance for catheter-based coronary interventions. J Am Coll Cardiol 1991;17:39B-45B. [Crossref] [PubMed]
- McCabe JM, Croce KJ. Optical coherence tomography. Circulation. 2012;126:2140-3. [Crossref] [PubMed]
- Secco GG, Micari A, Vadalà G, et al. Safety and efficacy of saline infusion for optical coherence tomography evaluation of vascular lesion induced by renal nerve ablation. Int J Cardiol 2013;168:5024-5. [Crossref] [PubMed]
- Secco GG, Grattoni C, Parisi R, et al. Saline vs contrast infusion during optical coherence tomography imaging of peripheral percutaneous intervention. Int J Cardiol 2014;172:246-8. [Crossref] [PubMed]
- Secco GG, Mattesini A, Fattori R, et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovasc Revasc Med 2016;17:38-43. [Crossref] [PubMed]
- Prati F, Regar E, Mintz GS, et al. Expert's OCT Review Document. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401-15. [Crossref] [PubMed]
- Secco GG, Grattoni C, Parisi R, et al. Optical Coherence Tomography Guidance during Peripheral Vascular Intervention. Cardiovasc Intervent Radiol 2015;38:768-72. [Crossref] [PubMed]
- Prati F, Cera M, Ramazzotti V, et al. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention 2007;3:365-70. [Crossref] [PubMed]
- Onuma Y, Serruys PW. Bioresorbable Scaffold: The Advent of a New Era in Percutaneous Coronary and Peripheral Revascularization? Circulation 2011;123:779-97. [Crossref] [PubMed]
- Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds: change in paradigm of coronary revascularization in the upcoming decade. Eur Heart J 2012;33:16-25b. [Crossref] [PubMed]
- Onuma Y, Dudek D, Thuesen L, et al. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv 2013;6:999-1009. [Crossref] [PubMed]
- Ellis SG, Kereiakes DJ, Metzger DC, et al. ABSORB III Investigators. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med 2015;373:1905-15. [Crossref] [PubMed]
- Woudstra P, Grundeken MJ, Kraak RP, et al. Amsterdam Investigator-initiateD Absorb strategy all-comers trial (AIDA trial): a clinical evaluation comparing the efficacy and performance of ABSORB everolimus-eluting bioresorbable vascular scaffold strategy vs the XIENCE family (XIENCE PRIME or XIENCE Xpedition) everolimus-eluting coronary stent strategy in the treatment of coronary lesions in consecutive all-comers: rationale and study design. Am Heart J 2014;167:133-40. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002;106:1640-5. [Crossref] [PubMed]
- Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol 2006;97:1172-5. [Crossref] [PubMed]
- Meng L, Lv B, Zhang S, et al. In vivo optical coherence tomography (OCT) of experimental thrombosis in a rabbit carotid model. Heart 2008;94:777-80. [Crossref] [PubMed]
- Kawasaki M, Bouma BE, Bressner J, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol 2006;48:81-8. [Crossref] [PubMed]
- Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058-72. [Crossref] [PubMed]
- Secco GG, Foin N, Viceconte N, et al. Optical coherence tomography for guidance of treatment of in-stent restenosis with cutting balloons. EuroIntervention 2011;7:828-34. [Crossref] [PubMed]
- Biscaglia S, Secco GG, Tumscitz C, et al. Optical coherence tomography evaluation of overlapping everolimus-eluting bioresorbable vascular scaffold implantation guided by enhanced stent visualization system. Int J Cardiol 2015;182:1-3. [Crossref] [PubMed]
- Biscaglia S, Ugo F, Ielasi A, et al. Bioresorbable Scaffold vs. Second Generation Drug Eluting Stent in Long Coronary Lesions requiring Overlap: A Propensity-Matched Comparison (the UNDERDOGS study). Int J Cardiol 2016;208:40-5. [Crossref] [PubMed]
- Biscaglia S, Campo G, Tebaldi M, et al. Bioresorbable vascular scaffold overlap evaluation with optical coherence tomography after implantation with or without enhanced stent visualization system (WOLFIE study): a two-centre prospective comparison. Int J Cardiovasc Imaging 2016;32:211-23. [Crossref] [PubMed]
- Gomez-Lara J, Diletti R, Brugaletta S, et al. Angiographic maximal luminal diameter and appropriate deployment of the everolimus-eluting bioresorbable vascular scaffold as assessed by optical coherence tomography: an ABSORB cohort B trial sub-study. EuroIntervention 2012;8:214-24. [Crossref] [PubMed]
- Serruys PW, Onuma Y, Ormiston JA, et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation 2010;122:2301. [Crossref] [PubMed]
- Onuma Y, Serruys PW, Muramatsu T, et al. Incidence and imaging outcomes of acute scaffold disruption and late structural discontinuity after implantation of the absorb Everolimus-Eluting fully bioresorbable vascular scaffold: optical coherence tomography assessment in the ABSORB cohort B Trial (A Clinical Evaluation of the Bioabsorbable Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv 2014;7:1400-11. [Crossref] [PubMed]
- Onuma Y, Sotomi Y, Shiomi H, et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention 2016;12:1090-101. [Crossref] [PubMed]
- Mattesini A, Secco GG, Dall'Ara G, et al. ABSORB biodegradable stents versus second-generation metal stents: a comparison study of 100 complex lesions treated under OCT guidance. JACC Cardiovasc Interv 2014;7:741-50. [Crossref] [PubMed]
- Fabris E, Caiazzo G, Kilic ID, et al. Is high pressure postdilation safe in bioresorbable vascular scaffolds? Optical coherence tomography observations after noncompliant balloons inflated at more than 24 atmospheres. Catheter Cardiovasc Interv 2016;87:839-46. [Crossref] [PubMed]
- Caiazzo G, Longo G, Giavarini A, et al. Optical coherence tomography guidance for percutaneous coronary intervention with bioresorbable scaffolds. Int J Cardiol 2016;221:352-8. [Crossref] [PubMed]
- Mattesini A, Boeder N, Valente S, et al. Absorb vs. DESolve: an optical coherence tomography comparison of acute mechanical performances. EuroIntervention 2016;12:e566-73. [Crossref] [PubMed]
- Boeder NF, Dörr O, Bauer T, et al. Impact of strut thickness on acute mechanical performance: A comparison study using optical coherence tomography between DESolve 150 and DESolve 100. Int J Cardiol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Seth A, Onuma Y, Costa R, et al. First-in-human evaluation of a novel poly-L-lactide based sirolimus-eluting bioresorbable vascular scaffold for the treatment of de novo native coronary artery lesions: MeRes-1 trial. EuroIntervention 2017;13:415-23. [Crossref] [PubMed]
- Haude M, Ince H, Kische S, et al. Sustained safety and clinical performance of aIn the drug-eluting absorbable metal scaffold up to 24 months: pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention 2017;13:432-9. [Crossref] [PubMed]
- Karjalainen P, Paana T, Sia J, et al. Neointimal Healing Evaluated by Optical Coherence Tomography after Drug-Eluting Absorbable Metal Scaffold Implantation in de novo Native Coronary Lesions: Rationale and Design of the Magmaris-OCT Study. Cardiology 2017;137:225-30. [Crossref] [PubMed]


