BRS implantation in long lesions requiring device overlapping: myth or reality?
ABSORB II, ABSORB III and AIDA: are three coincidences a proof?
ABSORB II
A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II) trial was the first prospective, randomized trial comparing bioresorbable vascular scaffold (BVS) and contemporary drug eluting stent (DES) in stable patients with relatively simple lesions (1). The aims of the study were to demonstrate the superiority in angiographic vasomotor reactivity after administration of intracoronary nitrate of BVS versus the Xience metallic stent and the non-inferiority with regard to late lumen loss both at 3 years. Absorb BVS was not superior in vasomotion and inferior in late lumen loss compared to Xience DES. Moreover, in the Absorb group there was a significant increase in target vessel-myocardial infarction (TV-MI) (6% vs. 1%; P=0.0108), mainly driven by peri-procedural MI (4% vs. 1%; P=0.16). In addition, during the 3 years follow-up, device definite/probable thrombosis was significantly higher in the Absorb than in the Xience group (9 vs. 0, P=0.0331).
The failure of the ABSORB II trial can be explained, at least in part, through several reasons. The utilization of angiographic vasomotion as primary endpoint of a trial is debatable. In the sample size, authors hypothesized to detect true changes in mean lumen diameter of 0.07 mm for the Absorb scaffold and 0 mm for the Xience metallic stent with a standard deviation (SD) 3 times higher than the supposed difference between groups (SD 0.20 mm for both), measured with quantitative coronary angiography (QCA). The low reliability of this endpoint is testified by the same results of the trial. BVS was not only non-superior to Xience in the vasomotion endpoint, in fact vasomotion was numerically inferior in the BVS group compared to Xience group (Absorb group 0.047 mm vs. Xience group 0·056 mm; P=0.49 for superiority). This is theoretically unlikely as the same authors hypothesized absence of vasomotion in the stented segment. A possible partial explanation of these results is that in human the reabsorption process may take longer than expected, as suggested by a recent case series focusing on very late scaffold thrombosis (2). In addition, vasomotion has never been demonstrated to be a surrogate marker of any clinical endpoint. Thus, it is questionable to base sample size on it and to use it as primary endpoint in the first randomized comparison involving a new device such as BVS. This limitation was partially overcome by the second co-primary endpoint, namely late lumen loss that has robust evidence as surrogate for clinical endpoints. However, a major technical concern in the late lumen loss analysis is represented by the inclusion of patients with scaffold thrombosis. This has clearly affected the results, since 8 patients had device thrombosis in the Absorb group while 0 in the Xience group. It is plausible that excluding those patients from the analysis the result would have been different. From a clinical point of view, the rate of scaffold thrombosis was worrisome and it has been confirmed by subsequent studies (3,4). A partial explanation of such a high scaffold thrombosis rate could be the suboptimal BVS implantation technique since all patients were randomized before the development of the predilation-sizing-postdilation (PSP) technique. Among the patients experiencing scaffold thrombosis, none was post-dilated with a non-compliant balloon with diameter ≥0.5 mm than scaffold.
ABSORB III
At the recent American College of Cardiology Meeting, the “Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease” (ABSORB III) trial 2-year results were presented. This was possible since after consult with Food and Drugs Administration, the landmark target lesion failure (TLF) endpoint was revised from between 1 and 5 years in the initial protocol to between 3 and 7 (or up to 10) years (3). This change allowed unblinding of clinical endpoints between 1 and 3 years in the ABSORB III trial. ABSORB III randomized 2,008 patients in a 2:1 fashion to receive BVS or Xience DES. Enrolled patients were mainly stable or with stabilized acute coronary syndromes (ACS) with simple lesions. The main result of the study was that patients receiving BVS had a significantly higher rate of TLF at 2 years (11% vs. 7.9%, P=0.03) driven by a significantly higher rate of TV-MI (7.3% vs. 4.9%, P=0.04). Although non significantly, scaffold thrombosis rate was higher in BVS arm compared to DES arm (1.9% vs. 0.8%, P>0.05). Authors showed that, taking into account only patients with reference vessel diameter (RVD) ≥2.25 mm at QCA, there was no significant difference between the two arms in terms of events. However, this was a sub-analysis and therefore merely speculative. Interestingly, a pooled analysis from ABSORB III-IV data showed that, in the overall ABSORB IV population, lowering the percentage of patients with QCA RVD <2.25 mm (19% in ABSORB III vs. 4% in ABSORB IV) and increasing the percentage of patients receiving postdilation (66% in ABSORB III vs. 83% in ABSORB IV), the overall rate of device thrombosis fell from 1.1% to 0.5% at 1 year. Obviously, we do not know the actual rate of scaffold thrombosis in the BVS group, but we can infer that it will be lower than the one showed by previous studies.
AIDA
Another important evidence is given by the results of the Amsterdam Investigator-Initiated Absorb Strategy All-Comers Trial (AIDA) recently published (4). The results were released due to the high rate of definite/probable scaffold thrombosis in the BVS group (3.5% at 2 years). AIDA was a nearly all-comers trial that enrolled 1,845 patients to BVS or DES at five high-volume percutaneous coronary intervention (PCI) centers in the Netherlands. The primary endpoint of TVF occurred was not different between the two groups (105 patients in the scaffold group vs. 94 patients in the stent group P=0.43). However, rates of TV-MI were significantly higher in in the scaffold group compared to the stent group (5.5% vs. 3.2%, P=0.04). The main concern of investigators was the number of definite or probable device thrombosis in the scaffold group (n=31) compared to the one of the stent group (n=8). The 2-year Kaplan-Meier event rates of definite or probable device thrombosis were 3.5% in the scaffold group and 0.9% in the stent group (P<0.001). These results are surely worrisome. Yet, it is worthy to note that among the 35 patients treated with BVS and experiencing a scaffold thrombosis in this trial, only 10 (29%) received a correct postdilation with a non-compliant balloon ≥0.5 mm scaffold size.
As a response to the multiplication of the above presented worrisome results with particular regard to the high rate of scaffold thrombosis, manufacturer decided to limit the use of BVS to experienced operators in high-volume centers applying the PSP (pre-dilatation, sizing to 1:1, post-dilatation with noncompliant balloon) technique in order to prospectively register data from a big population with standardized implantation technique. This will be extremely important in order to not put aside a new technology before having definitive data, remembering what has happened for first generation DES.
Adding irons in the fire: late clinical events and dual antiplatelet therapy (DAPT)
Once clarified the relevance of implantation technique on early clinical events, it is paramount to underline that recent evidences raise a red flag on late clinical events in patients receiving BVS. In a recent meta-analysis, very late device thrombosis occurred significantly more in patients treated with BVS compared to those treated with DES [12/996 (1.4%, 95% CI: 0.08–2.5) Absorb BVS vs. 1/701 (0.5%, 95% CI: 0.2–1.6) DES; OR 3.04; 95% CI: 1.2–7.68, P = 0.03] (5). There are two important considerations to be made. First, late events cannot be related to implantation technique. Second, 92% of the very late device thrombosis in the BVS group occurred in the absence of DAPT, suggesting that longer DAPT could be needed in patients receiving BVS. These results leave several unsolved issues: which is the mechanism of late events? Which is the proper DAPT duration in patients receiving BVS? There are actually no data able to solve these issue and further studies are clearly on demand.
Overlapping BVS: an impossible challenge?
The need of overlap implies a long and complex lesion in a complex patient and it is per se a high-risk setting. Previous studies regarding both first and second generation DES have shown that overlapping is associated with an increased rate of stent thrombosis and in-stent restenosis (6-8). A recent study evaluated the mechanism of post-procedural cardiac biomarker rise following everolimus-eluting metallic stent and BVS implantation. Authors showed no significant differences between the two devices with regard to cardiac marker rise. Unsurprisingly, overlap emerged as the main determinant of peri-procedural myocardial infarction (9).
Overlap between bioresorbable scaffolds (BRS) is particularly challenging, because of higher struts thickness and poor angiographic visualization of the scaffolds platform. Scaffold struts are at least 150 microns thick (156 microns for Absorb BVS and 150 microns for DESolve) in order to ensure adequate radial force to the scaffold (10,11). Actual second generation DES have a struts thickness of approximately 88 microns. Hence, when overlap is necessary, stacked struts are formed in the area of overlap with a thickness of more than 300 microns and a steric hindrance of the lumen >600 microns.
In a study executed in a porcine coronary artery model by Farooq et al. (12), overlapping BVS and Xience V were compared, and it was noted that endothelialization was delayed at 28 days (which correspond approximately to 6 months in humans) in BVS overlapping segments (both versus BVS non-overlapping segments and versus Xience V overlapping segments) (12). In the same study, it was also underlined that, at 90 days (which correspond to 18 months in humans), in the BVS group, the overlapping area showed a greater neointimal coverage compared to DES group (12).
These two findings in the porcine model might translate in higher rate of adverse events. Indeed, the delayed endothelialization in BVS overlapping area, as emerged from data obtained from some randomized clinical trials and registries, might be responsible of an increased rate of early scaffold thrombosis (13-15). Instead, there is no actual evidence establishing a correlation between increased neointimal response at 90 days and risk of in-scaffold restenosis (12). In view of foregoing assumptions, the consensus document of 2015 about the use of BVS in PCI, stated that the implantation of two scaffolds in overlap is probably appropriate (16).
Clinical data
A recent interesting manuscript by Geraci et al. (17), reported a sub-analysis of the GHOST-EU (Gauging coronary Healing with bioresorbable Scaffolding plaTforms in EUrope) registry on long coronary lesions treated with BVS. In their study the treatment of coronary lesions ≥60 mm with BVS was associated with a higher TLF rate if compared to treatment with shorter length of BVS (either ≤30 or 30–60 mm). Their results seem to raise major concerns regarding BVS implanted in overlap. However, there are several appraisals to be made. First, the subgroup of patients with the significantly higher rate of TLF events (≥60 mm) was composed by 81 patients. It is difficult to draw any conclusion on hard endpoints taking into account such a small population. Second, there were significant differences in baseline and procedural characteristics between the three groups with regard to diabetes mellitus II, ostial lesions and lesion length. All these characteristics emerged has predictors of adverse events at the multivariate analysis and were significantly more frequent in patients treated with ≥60 mm of BVS. Third, as showed above, this finding is not new. Overlap is a marker of high-risk, irrespectively from the utilized device, as recently showed for second generation DES (8). Thus, the difference in the outcome is attributable to patients and lesions complexity rather than to the use of a specific device. However, authors raise an important issue: the unacceptably high rate of scaffold thrombosis (3.8% at 1 year) in the ≥60 mm group. Their worrisome results are unfortunately in line with recently published and presented data (see above) (3,4). Still, it is noteworthy that we have no information on how overlap was performed in the study and no standard implantation technique was implemented.
Actually, we have no randomized comparison between overlapping DES and BVS. However, in the “Bioresorbable Scaffold vs. Second Generation Drug Eluting Stent in Long Coronary Lesions requiring Overlap: A Propensity-Matched Comparison” (UNDERDOGS) study (18), 162 patients receiving overlapping BVS were compared through a propensity score with 162 patients receiving overlapping DES. Implantation technique in the BVS group fully respected the PSP rule (98% of patients received predilation almost 1:1, 95% of patients received postdilation with a non-compliant balloon >0.5 mm scaffold size). The primary endpoint was the 1-year device oriented endpoint (DOCE) rate that resulted comparable between the two groups (5.6% in the BVS group vs. 7.4% in the DES group, P=0.50). Definite/probable device thrombosis rate was not different between the two groups (1.2% in the BVS group vs. 1.8% in the DES group, P=0.65). Interestingly, imaging techniques and/or enhanced stent visualization (ESV) systems were significantly more employed in the BVS group. This should be considered an indirect confirmation that operators are well aware that a careful and standardized BRS implantation technique is crucial to optimize the short- and long-term performance of this device.
Again, given the recent amount of worrisome results on BVS and the absence of randomized comparison, it is crucial to collect a greater body of evidence coming from big populations with standardized implantation technique. We have no randomized data regarding overlapping BVS versus contemporary DES. Thus, overlap is a delicate setting for BVS implantation. When overlap is needed, extreme attention should be paid to the implantation technique routinely applying the PSP technique and trying to minimize the overlap length as much as possible. The next section is dedicated to tips and tricks on how to optimize implantation when BVS overlap is needed.
Scaffold overlap implantation: technical aspects
PSP
Implantation is clearly more demanding in BRS than in second generation DES. It requires a more careful and meticulous approach, mainly due to the absence of a metallic cage, to the higher struts thickness and to the limit in postdilation. The most important technical aspects of BRS implantation are the following: (I) the appropriate sizing of the target vessel; (II) an adequate preparation of the lesion; and (III) adequate post-dilation of the scaffold with a non-compliant balloon with a diameter 0.5 mm greater than the size of the scaffold (the so called PSP) (19). A preliminary evidence of the clinical impact of PSP technique is given by a recent GHOST-EU sub-analysis in which patients with a maximum PSP score (meaning a correct implantation) had a significantly lower rate of DOCE at 1-year compared to other patients (19) and by the above mentioned pooled analysis from ABSORB III and IV (3). These steps are even more important long coronary lesions requiring overlap. First, sizing is crucial, given the lower margin for postdilation in BRS (only 0.5 mm). As showed by several studies (18,20), use of imaging techniques such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) is extremely helpful during BRS implantation, especially in the first part of the learning curve (20). Imaging is important both for correct sizing and for optimization of the procedure with postdilation.
Afterwards, the operator has to perform proper pre-dilatation with the aim to obtain a residual stenosis of less than 40%. Only at this point, it is possible to implant the scaffold. If overlap is needed, the operator has to position first the distal scaffold and, thereafter, the proximal one in order to avoid scaffold/balloon entrapment in the proximal scaffold with risk of struts disruption (21).
Once implanted the BVS, it is crucial to proceed to postdilation with non-compliant balloon, taking into account that BRS have an expansion limit of 0.5 mm, which must not be exceeded to avoid struts fracture or other complications and to check the result with imaging in order to avoid malapposition and ensure adequate scaffold expansion. It is worthy to note that, in a recent study, postdilation itself was able to improve outcome after BVS implantation (22).
How to perform overlap
Scaffold overlap patterns
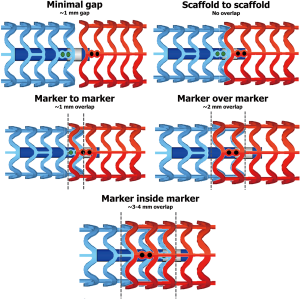
There are different pattern of overlapping scaffold: ‘minimal gap’, ‘scaffold to scaffold’, ‘marker to marker’, ‘marker over marker’, and ‘marker inside marker’ (Figures 1-3) (23,24).
- ‘Minimal gap’ consists in the positioning of the two scaffolds in order to precisely leave a minimal gap between the adjacent devices.
- ‘Scaffold to scaffold’ consists in a juxtaposition of the two scaffolds, through the positioning of the platinum markers side by side, without a real overlap of adjacent devices.
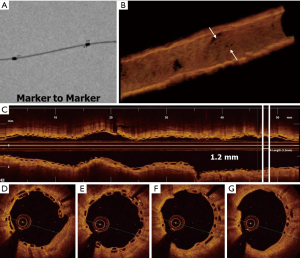
- ‘Marker to marker’ consists in positioning the distal marker of the proximal scaffold in correspondence with the proximal marker of the distal scaffold, achieving a minimum overlap (about 1 mm) with a reduced number of stacked struts.
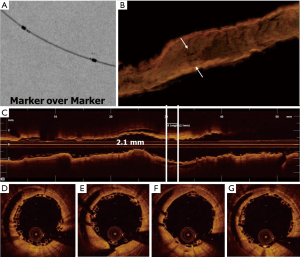
- ‘Marker over marker’ is obtained by the superimposition of the marker of the proximal scaffold with those of the distal one, obtaining a greater overlap (about 2 mm) with high number of stacked struts.
- ‘Marker inside marker’ is realized by positioning the proximal marker of the distal scaffold in correspondence with the distal marker of the distal scaffold with a very long overlap (3–4 mm).
Many authors agreed in defining the ‘marker to marker’ technique as the best to be pursued. In fact, with ‘marker over marker’ and ‘marker inside marker’ techniques there is a significant risk of stent thrombosis, due to the elevated number of stacked struts formed in overlapping area which might delay endothelialization, as suggested from Farooq et al. who tested different overlap patterns in a porcine model (23). On the other side, the ‘scaffold to scaffold’ and ‘minimal gap’ techniques heighten the risk of in-stent restenosis, because of ‘geographical miss’ (23).
Hence, the ‘marker to marker’ technique represents the best compromise, as it avoids leaving a gap between scaffolds, but at the same time creates a short overlap that does not delay struts coverage. Accordingly, in the UNDERDOGS study, the ‘marker to marker’ has been the most performed overlapping pattern (64% of cases) (18). All these considerations are referred to BVS but can be recommended also for DESolve, since material and struts thickness are similar to those of BVS. Indeed, in a recent observational study regarding DESolve, the ‘marker to marker’ method was performed when the overlap was required (25).
How to obtain a ‘marker to marker’ configuration
Although the ‘marker-to-marker’ is the most suitable overlap configuration to be pursued, it has been highlighted by several authors that its obtaining is substantially random and poorly reproducible when guided only by angiography. This is due to the fact that BRS are not radiopaque and therefore poorly visualized angiographically (26-29). Thus, it is advisable to perform the overlap between scaffolds with the use of ESV system usually integrated in most of angiographic systems (26-29). ESV system provides a magnification of radiopaque scaffold markers, allowing the operator can position scaffolds more accurately (26-29).
Conclusions
BRS are going through a very delicate moment given the worrisome results of several studies, particularly regarding scaffold thrombosis rate. However, these studies have several limitations, especially related to the implantation technique. The preliminary available data regarding BVS implanted according PSP technique seem to be reassuring (3,18,19).
However, bigger studies and registries enrolling patients receiving BRS with a standardized implantation technique are clearly on demand, especially in complex settings such as acute coronary syndrome or long lesions requiring overlap (30).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Räber L, Brugaletta S, Yamaji K, et al. Very Late Scaffold Thrombosis: Intracoronary Imaging and Histopathological and Spectroscopic Findings. J Am Coll Cardiol 2015;66:1901-14. [Crossref] [PubMed]
- Ellis SG, Kereiakes DJ, Stone GW. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results. Presented at the American College of Cardiology Annual Scientific Session (ACC 2017), Washington DC, March 18, 2017.
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med 2017. [E-pub ahead of print]. [Crossref] [PubMed]
- Collet C, Asano T, Miyazaki Y, et al. Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 2007;115:1440-55. [Crossref] [PubMed]
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126-30. [Crossref] [PubMed]
- Koskinas KC, Taniwaki M, Rigamonti F, et al. Impact of Patient and Lesion Complexity on Long-Term Outcomes Following Coronary Revascularization With New-Generation Drug-Eluting Stents. Am J Cardiol 2017;119:501-7. [Crossref] [PubMed]
- Ishibashi Y, Muramatsu T, Nakatani S, et al. Incidence and potential mechanism(s) of post-procedural rise of cardiac biomarker in patients with coronary artery narrowing after implantation of an everolimus-eluting bioresorbable vascular scaffold or everolimus-eluting metallic stent. JACC Cardiovasc Interv 2015;8:1053-63. [Crossref] [PubMed]
- Onuma Y, Serruys PW. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011;123:779-97. [Crossref] [PubMed]
- Okamura T, Garg S, Gutiérrez-Chico J, et al. In vivo evaluation of stent strut distribution patterns in the bioabsorbable everolimus-eluting device: an OCT ad hoc analysis of the revision 1.0 and revision 1.1 stent design in the ABSORB clinical trial. EuroIntervention 2010;5:932-8. [Crossref] [PubMed]
- Farooq V, Serruys P, Heo JH, et al. Intracoronary Optical Coherence Tomography and Histology of Overlapping Everolimus-Eluting Bioresorbable Vascular Scaffolds in a Porcine Coronary Artery Model The Potential Implications for Clinical Practice. JACC Cardiovasc Interv 2013;6:523-32. [Crossref] [PubMed]
- Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention 2015;10:1160-8. [Crossref] [PubMed]
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015;10:1144-53. [Crossref] [PubMed]
- Van Geuns RJ. BVS Expand: 6-month results. EuroPCR 2014. Available online: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides
- Everaert B, Felix C, Koolen J, et al. Appropriate use of bioresorbable vascular scaffolds in percutaneous coronary interventions: a recommendation from experienced users. A position statement on the use of bioresorbable vascular scaffolds in the Netherlands. Neth Heart J 2015;23:161-5. [Crossref] [PubMed]
- Geraci S, Kawamoto H, Caramanno G, et al. Bioresorbable Everolimus-Eluting Vascular Scaffold for Long Coronary Lesions: A Subanalysis of the International, Multicenter GHOST-EU Registry. JACC Cardiovasc Interv 2017;10:560-8. [Crossref] [PubMed]
- Biscaglia S, Ugo F, Ielasi A, et al. Bioresorbable scaffold vs. second generation drug eluting stent in long coronary lesions requiring overlap: a propensity-matched comparison (the UNDERDOGS study). Int J Cardiol 2016;208:40-5. [Crossref] [PubMed]
- Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: development and internal validation of the PSP score. EuroIntervention 2017;12:2110-7. [Crossref] [PubMed]
- Caiazzo G, Longo G, Giavarini A, et al. Optical coherence tomography guidance for percutaneous coronary intervention with bioresorbable scaffolds. Int J Cardiol 2016;221:352-8. [Crossref] [PubMed]
- Giblett JP, Brown AJ, Hoole SP, et al. Early disarticulation of a bioresorbable vascular scaffold: an underreported consequence of repeat imaging. Cardiovasc Interv Ther 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Imori Y, D'Ascenzo F, Gori T, et al. Impact of postdilatation on performance of bioresorbable vascular scaffolds in patients with acute coronary syndrome compared with everolimus-eluting stents: A propensity score-matched analysis from a multicenter "real-world" registry. Cardiol J 2016;23:374-83. [Crossref] [PubMed]
- Farooq V, Onuma Y, Radu M, et al. Optical coherence tomography (OCT) of overlapping bioresorbable scaffolds: from benchwork to clinical application. EuroIntervention 2011;7:386-99. [Crossref] [PubMed]
- Pichette M, Chevalier F, Genereux P. Coronary artery perforation at the level of two-overlapping bioresorbable vascular scaffolds: the importance of vessel sizing and scaffold thickness. Catheterization and Cardiovascular Interventions 2015;86:686-91. [Crossref] [PubMed]
- Blachutzik F, Boeder N, Wiebe J, et al. Overlapping implantation of bioresorbable novolimus-eluting scaffolds: an observational optical coherence tomography study. Heart Vessels 2017;32:781-789. [Crossref] [PubMed]
- Biscaglia S, Tumscitz C, Tebaldi M, et al. Enhanced stent visualization systems during PCI: A case series and review of literature. JCCASE 2015;588:1-5.
- Biscaglia S, Tebaldi M, Tumscitz C, et al. Prospective Identification of Stent Fracture by Enhanced Stent Visualization System During Percutaneous Coronary Intervention. Circ J 2016;81:82-9. [Crossref] [PubMed]
- Biscaglia S, Secco GG, Tumscitz C, et al. Optical coherence tomography evaluation of overlapping everolimus-eluting bioresorbable vascular scaffold implantation guided by enhanced stent visualization system. Int J Cardiol 2015;182:1-3. [Crossref] [PubMed]
- Biscaglia S, Campo G, Tebaldi M, et al. Bioresorbable vascular scaffold overlap evaluation with optical coherence tomography after implantation with or without enhanced stent visualization system (WOLFIE study): a two-centre prospective comparison. Int J Cardiovasc Imaging 2016;32:211-23. [Crossref] [PubMed]
- Ielasi A, Varricchio A, Campo G, et al. A prospective evaluation of a standardized strategy for the use of a polymeric everolimus-eluting bioresorbable scaffold in ST-segment elevation myocardial infarction: Rationale and design of the BVS STEMI STRATEGY-IT study. Catheter Cardiovasc Interv 2017;89:1129-1138. [Crossref] [PubMed]