Predictors of conversion to thoracotomy during video-assisted thoracoscopic surgery lobectomy in lung cancer: additional predictive value of FDG-PET/CT in a tuberculosis endemic region
Introduction
Surgical resection is the primary mode of treatment for early non-small cell lung cancer. Video-assisted thoracoscopic surgery (VATS) has been considered the initial approach for the majority of patients undergoing lobectomy, since its introduction in the 1990s. Although some potential hazards have been reported, recent studies have shown that VATS lobectomy could have more advantages than lobectomy via thoracotomy with regard to postoperative pain (1), postoperative rehabilitation (2), preservation of pulmonary function (3), and even surgical complications and cost (4). Despite these advantages, only 30–40% of anatomic lung resections were performed via the VATS approach according to the thoracic database (5,6). One of the reasons that prevent more widespread acceptance of VATS lobectomy is a potentially higher risk of intraoperative complication and subsequent unplanned conversion to thoracotomy during the surgery.
Several demographic and clinical factors such as older age, history of the granulomatous disease, current COPD or smoker, and reduced forced expiratory volume in 1 second (FEV1) have been identified to be predictors of intraoperative conversion (7-9). Additionally, recent studies revealed that computed tomography (CT) images with hilar lymph node calcification could predict intraoperative conversion, which was associated with adhesion of lymph nodes to hilar structures and subsequent difficulty in the dissection of hilum (8-10). However, even lymph nodes without calcification on CT images revealed extensive perihilar granulomatous inflammation during the operation, which required conversion to thoracotomy (9,10). Using 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT, noncalcified lymph nodes with high FDG uptake have been associated with chronic granulomatous or anthracofibrotic pathology of lymph nodes when they show attenuation higher than that of surrounding great vessels, especially in an endemic region for tuberculosis (11,12). These findings prompted our hypothesis that the metabolic information of FDG PET/CT might better predict hilar anthracofibrosis leading to conversion to thoracotomy in lung cancer operations when added to the morphologic information of chest CT.
Therefore, we conducted this study to verify the added clinical value of FDG PET/CT imaging in comparison with chest CT imaging alone for predicting the conversion to thoracotomy during thoracoscopic surgery in lung cancer patients. We also evaluated the association between conversion status and other CT characteristics associated with previous granulomatous disease, including pleural calcification and parenchymal calcified nodule, to identify the predictive imaging features for the conversion to thoracotomy.
Methods
The study was approved by our institutional ethics committee of Kyungpook National University Medical Center (KNUMC 2016-11-003), and the requirement of informed consent was waived for this retrospective analysis.
Patients
All patients diagnosed with primary lung cancer from February 2011 to May 2015 and who had undergone lobectomy at Kyungpook National University Medical Center were recruited for this study. One experienced surgeon (L.E.B) with 18-years of thoracic surgical experience performed the VATS surgery. During the study period, 354 patients underwent surgery. We excluded those with a planned thoracotomy, those with a sublobar resection through VATS, those with a history of neoadjuvant chemotherapy or radiotherapy, and those with a history of prior thoracic surgery. We also excluded 53 patients with hilar metastatic lymphadenopathy based on the pathological exam. Finally, 235 patients were selected for the current study, among whom, 55 (23.4%) underwent conversion to thoracotomy. The pathological stage was determined by applying the seventh edition of the Union for International Cancer Control and American Joint Committee on Cancer TNM classification (13).
Surgical technique
All patients underwent single lung ventilation with a double-lumen endotracheal tube. Details of surgical techniques for VATS lobectomy were as follows. A 15 mm trocar for the thoracoscope and a 4 to 5 cm utility incision were placed at the level of the top of the diaphragm, crossing the anterior axillary line and posterior scapular line. The additional 5 to 10 mm port was positioned through sixth or seventh intercostal space in the posterior scapular line. Conversion to open thoracotomy was performed by extending the utility incision or by linking two incisions.
Imaging acquisition
CT scans were performed with two 64-channel multi-detector CT (MDCT) systems (GE Medical Systems, Milwaukee, WI, USA). Both unenhanced and contrast-enhanced images were acquired. For the enhancement study, intravenous contrast material was administered (80 to 120 mL at a rate of 2.5 mL/sec). The technical parameters were as follows: tube voltage, 120 kVp; pitch, 1.25; rotation speed, 0.5 seconds; collimation, 64×0.625 mm. The axial images were obtained with 1.0–5.0 mm from the lung apices to bases at the end of an inspiration and reconstruction thicknesses were 2.5–5 mm. All PET/CT studies were performed with a 16-slice CT Discovery STE apparatus® (GE Medical System, Milwaukee, WI, USA). After fasting for at least six hours before the PET/CT examination, approximately 5.2 MBq of FDG/kg of body weight was injected intravenously, and then patients were advised to rest for one hour before image acquisition. Prior to PET/CT, CT imaging was performed from the skull base to the thigh when the patient was supine and breathing quietly according to a standard protocol with the following parameters: 120 KeV; 30–170 mA; 1.5 pitch; 15 mm/rotation table speed; and a 3.75mm slice thickness. PET scans were also obtained from the skull base to the thigh at 2.5 minutes per bed position. CT and PET/CT scan data were collected from a picture archiving and communication system (PACS; Infinitt Healthcare Co. Ltd., Seoul, Korea). All CT scans and integrated PET/CT imaging were performed within 2 weeks of surgery prior to surgery.
Image interpretation
Two radiologists who were blinded to the patient`s conversion status retrospectively reviewed the chest CT and FDG PET/CT scans, and a consensus diagnosis was reached. CT images were interpreted in terms of the presence of peribronchial or perivascular lymph nodes (PLN) and peribronchial cuffs of soft (PCS) tissue where the lung cancer was located. PCS was defined as peribronchial wall thickening with soft tissue attenuation at both sides of the bronchus on axial images (14,15). The lymph nodes were evaluated in terms of their short-axis diameter on the axial CT images and the attenuation of PLN or PCS was also reviewed. Calcification was considered present when it was nodular or diffuse and attenuation was 200 HU or greater. A high-attenuation was defined as one that appeared to have attenuation higher than that of mediastinal vascular structures and 70 HU or greater according to region of interest (ROI) measurement. The CT findings of pleural calcification and parenchymal calcified nodule were also evaluated as image characteristics for predicting pleural adhesion and subsequent conversion. Pleural calcification was defined as any calcification at the pleural area on the axial images of unenhanced CT. Any size of parenchymal nodule containing calcification on the unenhanced CT images was regarded as parenchymal calcified nodule, regardless of the shape of calcification (16,17). Calcification was defined as the attenuation above 200 HU and measured in mediastinal window setting (window with, 400 HU; window center, 30 HU). On FDG PET/CT images, the diagnostic criteria for anthracofibrotic lymph node was defined as when high FDG uptake (higher than that of the surrounding normal mediastinal structure, or a maximum standardized uptake value (SUVmax >3.5) was observed on PET/CT images corresponded to PLN or PCS on chest CT.
Statistical analysis
Statistical analyses were performed using statistics software (SPSS, version 17.0, SPSS, Inc., Chicago, Illinois, USA) and MedCalc for Windows, Version 14.8.1 (MedCalc Software, Mariakerke, Belgium). We compared the differences in CT findings and clinical characteristics between conversion and nonconversion groups with independent t-tests for continuous variables and with linear-by-linear test for ordinal variables, and used the chi-square or Fisher`s exact test for categorical variables. Logistic regression analysis was used to identify the predictive factors for VATS conversion to thoracotomy. CT and clinical variables with p values <0.05 in the univariate analyses were included in the multivariate analyses. ROC curve was illustrated with the predicted probability obtained from the logistic regression model, and the area under curve (AUC) was used to assess the additional predictive value of FDG PET/CT imaging in comparison with chest CT imaging alone. Statistical significance of the improvement in AUC after adding an explanatory factor was evaluated by the Delong test (18).
Results
Patient characteristics and imaging features
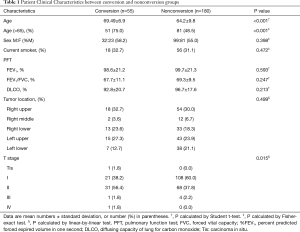
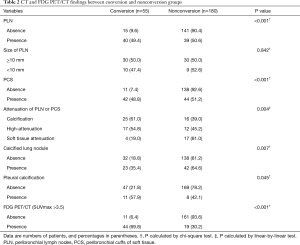
The clinical characteristics of patients in terms of the status of conversion are summarized in Table 1. The age and tumor stage of conversion group patients were significantly higher compared with those of patients undergoing successful VATS. There was no statistical difference in sex, smoking pack-years, spirometry results, and lobar location of lung cancers between conversion and non-conversion groups (Table 1). With respect to CT and FDG PET/CT findings, the conversion group had a higher proportion of PLN, PCS, pleural calcification, calcified lung nodule, calcification or high attenuation of PLN or PCS on chest CT and concurrent FDG uptake at PET/CT (Table 2). PLNs with calcification resulted in a highest rate of conversion (25/41, 61.0%), followed by those with high attenuation (17/31, 54.8%), and those with soft tissue (4/21, 19.0%). When these lymph nodes showed concurrent FDG uptake at PET/CT, the conversion rate was increased to 24/28 (85.7%), 16/22 (72.7%), and 4/10 (40%), respectively.
Full table
Full table
Predictive factors for thoracotomy conversion
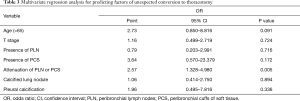
Among the 235 patients undergoing attempted VATS lobectomy, 55 patients (23.4%) underwent conversion to thoracotomy. The causes of conversion were as follows: severe anthracofibrosis around bronchus or vessels in 25 (45.5%), uncontrolled bleeding due to intraoperative vascular injury in 12 (21.8%), diffuse dense pleural adhesion in 9 (16.4%), incomplete or fused interlobar fissure in 7 (12.7%), and failure of single-lung ventilation in 2 patients (3.6%). We performed the multivariate logistic regression analysis for clinical and CT variables that were significantly associated with conversion status in the univariate analysis. After adjusting for other clinical and imaging features, the attenuation of PLN or PCS on chest CT scans (OR, 2.57; 95% CI, 1.328–4.980, 0.005) was an only independent predictor for conversion (Table 3).
Full table
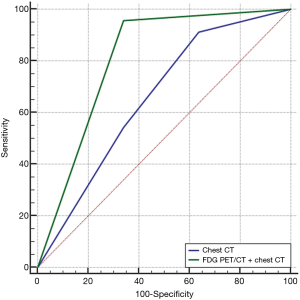
The ROC curve was illustrated with the predicted probability obtained from the logistic regression to compare the predictive value of chest CT scans alone with a combination of chest CT and FDG PET/CT scans. As shown in Figure 1, the AUC of the FDG PET/CT and chest CT combination [0.847 (95% CI, 0.795–0.891); sensitivity, 80.00; specificity, 89.44; P value <0.001] for the predictive performance of conversion was significantly better than that of chest CT alone [0.655 (95% CI, 0.550–0.751); sensitivity, 91.30; specificity, 36.17; P value =0.003], (difference of AUC =0.153, P value=0.024, as assessed by DeLong test). This result suggests the additive role of FDG uptake of PLN or PCS at PET/CT imaging in predicting conversion to thoracotomy during VATS lobectomy.
Discussion
We conducted this study to identify whether FDG PET/CT scans could provide added predictive value to CT scans for intraoperative conversion of VATS lobectomy for lung cancer in a tuberculosis endemic region. Multivariate analysis revealed that attenuation of PLN or PCS (calcification or high attenuation) was an only independent predictive factor for conversion on CT. In addition, ROC curves showed that a combination of FDG PET/CT and CT scans had better performance than CT scans alone for predicting conversion, suggesting that FDG PET/CT imaging would help enhance detecting peribronchial or perivascular fibrosis complicating hilar dissection during VATS lobectomy and ultimately requiring intraoperative conversion.
Intraoperative conversion to thoracotomy during VATS lobectomy does not appear to compromise prognosis. However, it is likely to result in a longer operating time, extra lung manipulation, and increased blood loss leading to adverse surgical outcomes (6). To avoid these post-operative complications, it is imperative to identify the preoperative risk factors to reduce unexpected thoracotomy conversion. There are several studies evaluating the role of imaging studies in predicting conversion, and they revealed that hilar lymph node calcification on preoperative chest CT is an important factor for predicting conversion (8-10). They evaluated the relationship between the presence of calcified lymph node and conversion, but they did not describe other CT findings with regard to peribronchial fibrosis. In the present study, we also evaluated the presence of PCS tissue that was identified to be associated with peribronchial fibrosis with anthracotic pigmentation (15). In addition, we included the attenuation and size of peribronchial lymph nodes (PLN) as reviewed imaging features and found that high attenuation, as well as calcification, was significantly associated with conversion irrespective of the size of PLN. After adjusting for age, tumor stage, and other CT features associated with previous granulomatous disease (pleural calcification and parenchymal calcified nodule), the attenuation of PLN or PCS was an independent CT predictor for conversion. Moreover, we identified that additional FDG uptake of PLN or PCS on PET/CT scans provided better performance than chest CT scans alone for predicting conversion.
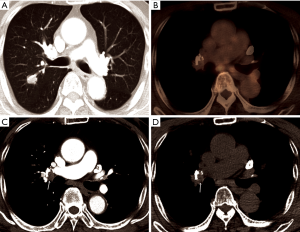
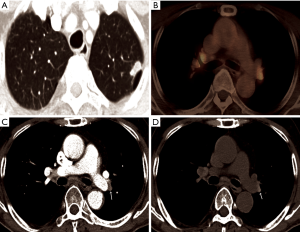
Where the granulomatous disease is endemic, hilar or mediastinal lymph nodes show follicular hyperplasia in the cortex, anthracotic pigmentation, and macrophage infiltration. This follicular hyperplasia and macrophage infiltration may contribute to increased FDG uptake. In the present study, of 41 patients with calcified PLN or PCS, the conversion was conducted in only 25 patients (61.0%), while, in patients with PLN or PCS calcification and concurrent high FDG uptake, most patients (24/28, 85.7%) underwent conversion. Conversely, almost all patients (12/13, 92.3%) with PLN or PCS but without FDG uptake at PET/CT, even when it showed calcification, completed VATS lobectomy (Figure 2). According to previous studies, hilar calcification on preoperative CT scans has been regarded as a convincing predictor for conversion due to peribronchial anthracofibrosis (8-10). However, our data highlight that some patients with calcified PLN or PCS may be suitable candidates for VATS lobectomy as long as they do not have concurrent FDG uptake. In other words, additional FDG PET uptake may provide more specific predictive information for peribronchial adhesion than hilar calcification only. Therefore, some patients who have calcification only (without significant FDG uptake on PET/CT) did not show significant peribronchial adhesion complicating hilar dissection. Moreover, in patients with high attenuation and soft tissue attenuation, the conversion rate increased from 54.8% and 19.0% to 72.7% and 40.0%, respectively, when they had additional FDG uptake (Figure 3). The ROC curve comparing CT scans alone and combined CT and FDG PET/CT scans for predicting conversion revealed that additional FDG PET/CT had better predictive performance than CT alone, particularly by improving specificity, despite a moderate decrease of sensitivity (Figure 1). Based on these observations, it can be assumed that additional FDG uptake at PET/CT scans enabled detection of more severe peribronchial and perivascular granulomatous inflammation and subsequent fibrosis requiring conversion by adding metabolic information to the morphological information of CT scans. These hilar anthracofibrotic changes may cause adhesion of lymph nodes to hilar structures and consequently result in the increased risk of vascular injury during the dissection of hilar structures, ultimately requiring conversion to thoracotomy.
In the present study, the most common cause of conversion was peribronchial or perivascular adhesion (25/55, 45.5%), which is consistent with what has previously been reported (8,10,19,20). The second cause of conversion was bronchial or vascular injury (12/55, 21.8%) that frequently lead to uncontrolled bleeding from pulmonary vessels. Given that a sizable number of bronchial or vascular injuries were attributed to hilar adhesion, our results demonstrate that hilar adhesion can be a major cause of conversion during VATS. The conversion rate in the present study was 23.4%, which is higher than that of published data (2–23%) (2,9,21,22). There are several possible explanations for this result. First, the current study was performed in a region of endemic for tuberculosis, which could account for a high prevalence of anthracofibrotic lymph nodes and subsequent hilar adhesion. Second, anthracofibrosis occurs predominantly in elderly patients at a mean age of 70 years (14,23). Since the study population of the current study had a higher mean age compared with those of other reports (7-9), this might have contributed to a higher incidence of hilar anthracofibrosis. Lastly, the selection criteria for VATS lobectomy candidates are most likely to affect the higher conversion rate. In our institution, patients with pleural calcification or calcified lymphadenopathy on preoperative CT scans were considered as potential VATS lobectomy candidates even though similar patients had been excluded from being VATS lobectomy candidates in other studies (8,10). Previous reports have shown that conversion rate depended on the surgeon`s experience and with increasing surgical experience, conversion rates declined (7,24). Given that the surgeries in the current study were performed by an experienced surgeon, our data may be more reliable than those from studies where the surgeries would be performed by several surgeons with varying levels of surgical expertise.
There are some limitations to the present study that should be considered. First, since this study is retrospective in nature, there may have been a selection bias in allocating surgical approaches between thoracotomy and VATS lobectomy. However, VATS candidates were selected consistently by one experienced surgeon in the practical preoperative decision.
Second, patients with hilar lymph node metastases were excluded from the analysis because our main concern was to evaluate the influence of peribronchial anthracofibrotic lymph nodes on conversion. Actually, the differential diagnosis between benign anthracofibrotic and metastatic lymph nodes when they show FDG uptake at PET/CT is difficult even in lymph nodes with high attenuation or calcification (11), which limits the evaluation of the sole impact of anthcofibrotic lymph node. Additionally, the conversion rate of excluded patients with nodal metastasis (13/53, 24.5%) was similar to that of the included patients and the presence of metastatic lymph node was not associated with the conversion in the previous studies (7,8).
In conclusion, the addition of FDG PET/CT to routine chest CT improves predictive performance for intraoperative conversion to thoracotomy during VATS lobectomy in lung cancer patients. Therefore, in lung cancer patients undergoing surgical resection, FDG PET/CT imaging may provide additional information in selecting the appropriate surgical approach for a lobectomy.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional ethics committee of Kyungpook National University Medical Center (KNUMC 2016-11-003) and the requirement of informed consent was waived for this retrospective analysis.
References
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Nomori H, Ohtsuka T, Horio H, et al. Difference in the impairment of vital capacity and 6-minute walking after a lobectomy performed by thoracoscopic surgery, an anterior limited thoracotomy, an anteroaxillary thoracotomy, and a posterolateral thoracotomy. Surg Today 2003;33:7-12. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 2 e1.
- Byun CS, Lee S, Kim DJ, et al. Analysis of unexpected conversion to thoracotomy during thoracoscopic lobectomy in lung cancer. Ann Thorac Surg 2015;100:968-73. [Crossref] [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [Crossref] [PubMed]
- Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology 2005;236:1011-9. [Crossref] [PubMed]
- Kwon SY, Min JJ, Song HC, et al. Impact of lymphoid follicles and histiocytes on the false-positive fdg uptake of lymph nodes in non-small cell lung cancer. Nucl Med Mol Imaging 2011;45:185-91. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Kahkouee S, Pourghorban R, Bitarafan M, et al. Imaging Findings of Isolated Bronchial Anthracofibrosis: A Computed Tomography Analysis of Patients With Bronchoscopic and Histologic Confirmation. Arch Bronconeumol 2015;51:322-7. [PubMed]
- Chung MP, Lee KS, Han J, et al. Bronchial stenosis due to anthracofibrosis. Chest 1998;113:344-50. [Crossref] [PubMed]
- Jin KN, Sung YW, Oh SJ, et al. Association between Image Characteristics on Chest CT and Severe Pleural Adhesion during Lung Cancer Surgery. PLoS One 2016;11:e0154694. [Crossref] [PubMed]
- Mason AC, Miller BH, Krasna MJ, et al. Accuracy of CT for the detection of pleural adhesions: correlation with video-assisted thoracoscopic surgery. Chest 1999;115:423-7. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Li Y, Wang J, Yang F, et al. Indications for conversion of thoracoscopic to open thoracotomy in video-assisted thoracoscopic lobectomy. ANZ J Surg 2012;82:245-50. [Crossref] [PubMed]
- Pischik VG. Technical difficulties and extending the indications for VATS lobectomy. J Thorac Dis 2014;6:S623-30. [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [Crossref] [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Video-assisted thoracoscopic major pulmonary resections: technical aspects, personal series of 259 patients, and review of the literature. Surg Endosc 2004;18:1551-8. [PubMed]
- Kim YJ, Jung CY, Shin HW, et al. Biomass smoke induced bronchial anthracofibrosis: presenting features and clinical course. Respir Med 2009;103:757-65. [Crossref] [PubMed]
- Gazala S, Hunt I, Valji A, et al. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:962-4. [Crossref] [PubMed]


