Uniportal video-assisted thoracoscopic right upper posterior segmentectomy with systematic mediastinal lymphadenectomy
Introduction
Over the past decade, double- or multiple-port video-assisted thoracoscopic surgery (VATS) lobectomy has become the first choice for early-stage non-small-cell lung carcinoma (1). However, lobectomy can also be accomplished with a single incision (uniportal), with potentially better cosmesis and reduced access trauma. Uniportal access was initially described by Rocco and colleagues for minor thoracic and pulmonary procedures (2,3). Gonzalez Rivas first reported the use of uniportal VATS lobectomy, which became a milestone in the history of VATS lobectomy development (4). It has now become an increasingly popular approach for managing thoracic surgical diseases. Moreover, uniportal VATS has evolved into a sophisticated technique that can be used to perform some of the most complex thoracic procedures, such as anatomic segmentectomy, sleeve lobectomy, and pulmonary artery reconstruction (5-7). However, the uniportal VATS approach to segmentectomy is not standardized, and the surgical procedure varies between surgeons during segmentectomy. This article presents our surgical experience with right upper posterior segmentectomy for systematic lymphadenectomy via uniportal VATS.
Clinical summary
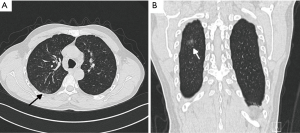
A ground-glass opacity (GGO) lesion had been identified at the right upper lobe in a 58-year-old man during incidental computed tomography (CT) screening 3 months prior to his presentation to us. Before admission, the patient underwent additional CT screening, and it indicated that the lesion had not significantly changed. The patient underwent bronchoscopy with no significant positive findings, but he refused a CT-guided biopsy. CT imaging defined an 18-mm solitary GGO nodule at the posterior segment (S2) of the right upper lobe (Figure 1). The patient denied any other medical history. He was also assessed preoperatively to ensure that there was no evidence of metastatic disease.
Operative techniques
Prior to the operation, the lesion location and the anatomy of the branches of the bronchus, arteries, and veins were defined using axial and coronal views of high-resolution CT. When we had confirmed that the lung could be resected at least 2 cm away from the tumor during segmentectomy, we proceeded with the uniportal VATS S2-segmentectomy. The surgical procedure is described below (Figure 2).

Anesthesia, positioning, and port placement
The patient was placed in the left lateral decubitus position with the arms extended to 90°. To protect the intercostal neurovascular bundles, the table was flexed to maximize the intercostal spaces. General anesthesia was induced, and intubation was achieved via a double-lumen endobronchial tube. The positions of the surgeon and the assistant were as described previously (9). The incision, about 3 cm long, was performed in the 5th intercostal space in the anterior position. A 3.5-cm plastic wound protector kept the utility wound open.
Right S2 segmentectomy
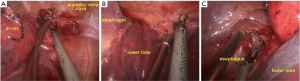
The hilum of the upper lobe was exposed from the ventral to the dorsal side to reveal the truncus superior artery, and the artery was dissected distal to expose the recurrent artery (Rec.A2, Figure 3A). The Rec.A2 was then transected via a stapler (Covidien Inc., Mansfield, MA, USA). As the lesion at S2 was close to the oblique fissure, in order to obtain a sufficient margin for resection, the parenchyma was pulled cephalad as we were stapling the incomplete oblique fissure to ensure that part of the superior segment adjacent to the oblique fissure could also be removed. The central vein was visualized after dividing the fissure and exposed in the peripheral direction. After branching the V3a, the V2 was ligated and transected (Figure 3B). Then, the posterior segmental artery (Asc.A2) was exposed, dissected, and ligated (Figure 3C). The 12th group lymph nodes were peeled from the right upper lobar bronchus. The tissues overlying its superior aspect were freed forward until the posterior segmental bronchus (B2) became apparent. The B2 was then transected by stapler with the guidance of a 16F urinary catheter (Figure 3D).
Before dividing the parenchyma, we reventilated the right lung. When the right lung, including S2, was completely inflated, we stopped ventilation and waited for the right lung to collapse. After 5 minutes, however, the S2 was still inflated. The fissure could then be easily divided along the inflation-deflation line using the stapler (Figure 3E). When the segmentectomy was completed, the S2 was removed in a protective bag through the utility incision.
Radical mediastinal lymphadenectomy
Examination of a frozen section confirmed lung adenocarcinoma with a lipidic-predominant tumor with <5 mm invasion; systematic mediastinal lymphadenectomy was then performed.
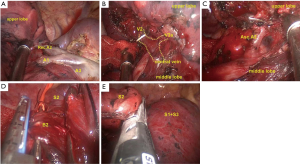
After opening the pleura inferiorly to the azygos vein, lifting the azygos vein, and retracting the superior vena cava to the right side by a suction device, the paratracheal lymph nodes (2nd and 4th group) were dissected en bloc (Figure 4A). Upon retracting the lobe with a long-curved grasper, the 8th and 9th group lymph nodes near the inferior pulmonary ligament were dissected (Figure 4B). Then, the lung was pushed forward by the assistant. The posterior mediastinal pleura was opened in front of the right vagus nerve, from the right inferior pulmonary vein to the azygos vein. After the esophagus and the intermediate bronchus were separated to facilitate the procedure, the right subcarinal lymph node (7th group) was dissected en bloc (Figure 4C).
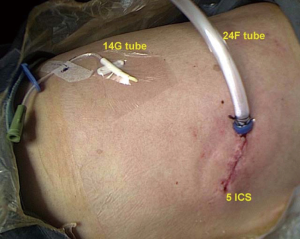
Finally, the lung was re-expanded for air leakage testing from the bronchial stump and pulmonary resection margin. A single 24F chest tube, which was connected to a negative pressure drainage bottle, was placed in the posterior part of the incision up to the tip of the thoracic cavity prior to closure of the port site. At the same time, we place a 14G fine tube in the intercostal space inferior to the incision, which was connected to a drainage bag (Figure 5).
Postoperative management
The operative time was 160 min. The time of S2 segmentectomy was 70 min, and the lymph node dissection time was 35 min. The number of lymph nodes dissected was 24, and the perioperative blood loss was 100 cc. The patient was observed for 1 day in the intensive care unit, and postoperative drainage continued for 3 days. The 24F tube was removed on the first day after surgery because there was no obvious gas overflow from the tube and plain radiography of the chest showed lung re-expansion. The fine tube was removed on the day of discharge when the daily drainage was <200 cc. The postoperative hospitalization time was 4 days. There were no complications, and the final pathology revealed a T1N0M0 minimally invasive lung adenocarcinoma.
Comments
At present, uniportal VATS is indicated not only for initial disease stages or easy cases but also has evolved into a sophisticated technique that can be used to perform some of the most complex thoracic procedures in the same manner as a double- or multiple-port approach (1,10). However, there are still some detailed changes for the surgical procedure in comparison to the classical multiple-port approach.
Unlike triple- or double-port techniques, which can dissect and isolate pulmonary vessels or the bronchus from different angles, it may be difficult to use single-port lobectomy when performing some complex procedures due to the utility angle of view via this approach (11). In this case, we used a 16F urinary catheter as a guide for passing the endostapler anvil around the B2. We used a thread to pull the end of the catheter through the gap, and then pushed the endostapler anvil into the gap without additional work. This procedure avoided the insertion of an excessive number of instruments simultaneously and damage to vessels, which run behind the bronchus.
Before division of the parenchyma, recognition of the intersegmental plane could be accomplished by differential inflation via reventilation (5). In this case, we used a modified method that helped us detect the actual intersegmental plane during segmentectomy. After the bronchovascular elements were controlled and divided when the right lung was deflated, the right lung was then completely re-inflated by reventilation with pure oxygen. The targeted segment could also be inflated by air transmission via the intersegmental communication. Right lung ventilation was then stopped. In this procedure, the parenchyma should not be compressed; the right lung should collapse naturally. After 5–10 minutes, we found the intersegmental line between the inflated target segment and the collapsed preserved segments. We believe that, because of the lack of blood flow, the pure oxygen in the target segment could not be absorbed. However, the preserved segments could absorb alveolar oxygen and collapse due to the non-blocking blood flow. Although the operation time was increased, this technique is simple and accurate, allowing clear visualization of the intersegmental plane from the physiology between the two segments.
A conventional chest drainage tube (24F) is one of the primary causes of postoperative pain (12). A fine tube causes almost no obvious pain when placed, but it drains air poorly. Therefore, we made some improvements to it. Before the port site was closed, we placed two chest tubes (24F and 14G) simultaneously. On the first day after surgery, if there was no obvious gas overflow from tube, plain radiography of the chest showed lung re-expansion, and the drainage fluid was not bloody, we removed the 24F chest tube. The fine tube was removed on the day of discharge. This procedure will not only relieve postoperative pain and inconvenience, but will also reduce hospital stay.
In our experience, based upon the evolution from multiple-port to single-port VATS, we have applied the single-port technique mostly for lobectomies and segmentectomies. We believe that the uniportal approach could be an easy and standardized approach for even the most complex thoracic procedures at centers with consistent experience with multiple-port VATS.
Acknowledgements
Dr. Gang Shen was awarded “The Master of Thoracic Surgery” and was granted the Award of Great Potential in the 2016 Masters of Thoracic Surgery—Uniportal VATS Lobectomy & VATS Segmentectomy Video Contest. Funding: This work was supported by grants from the Science and Technology Department of Zhejiang Province (2013c03044-7).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient.
References
- Migliore M. Initial History of Uniportal Video-Assisted Thoracoscopic Surgery. Ann Thorac Surg 2016;101:412-3. [Crossref] [PubMed]
- Rocco G, Khalil M, Jutley R. Uniportal video-assisted thoracoscopic surgery wedge lung biopsy in the diagnosis of interstitial lung diseases. J Thorac Cardiovasc Surg 2005;129:947-8. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Mendez L, Delgado M, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy. J Thorac Dis 2013;5 Suppl 3:S226-33. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [PubMed]
- Gonzalez-Rivas D, Aymerich H, Bonome C, et al. From Open Operations to Nonintubated Uniportal Video-Assisted Thoracoscopic Lobectomy: Minimizing the Trauma to the Patient. Ann Thorac Surg 2015;100:2003-5. [Crossref] [PubMed]
- Zhang G, Wu Z, Wu Y, et al. Uniportal video-assisted thoracoscopic right upper posterior segmentectomy with systematic mediastinal lymphadenectomy. Asvide 2017;4:416. Available online: http://www.asvide.com/articles/1730
- Shen G, Chai Y, Huang L, et al. Uniportal video-assisted thoracoscopic right upper lobectomy with systematic lymphadenectomy. J Thorac Dis 2016;8:2275-80. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Ng C. Advances in Uniportal Video-Assisted Thoracoscopic Surgery: Pushing the Envelope. Thorac Surg Clin 2016;26:187-201. [Crossref] [PubMed]
- Cheng K, Zheng B, Zhang S, et al. Feasibility and learning curve of uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2016;8:S229-34. [PubMed]
- McElnay PJ, Molyneux M, Krishnadas R, et al. Pain and recovery are comparable after either uniportal or multiport video-assisted thoracoscopic lobectomy: an observation study. Eur J Cardiothorac Surg 2015;47:912-5. [Crossref] [PubMed]