Interstitial lung disease: the diagnostic role of bronchoscopy
Introduction
Interstitial lung diseases (ILDs), also known as diffuse parenchymal lung diseases (DPLDs), encompass a wide and diverse group of disorders affecting not only the interstitium, but also peripheral airways, alveoli, and small blood vessels to a varying degree. They combine diseases of known (environmental, drug related, and collagen vascular diseases) and unknown etiologies [idiopathic interstitial pneumonia (IIPs), sarcoidosis, and others] (Figure 1) (1-5).
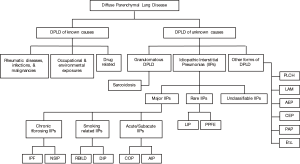
These entities share clinical, radiographic, physiologic, and pathologic manifestations, thus raising a challenge to making the correct diagnosis. A detailed history (with focus on exposure and systemic manifestations), and a complete physical exam are crucial, especially prior to making a diagnosis of IIPs. In clinical practice, patients can be easily misclassified as having an IIP when a medical history is not optimally taken. A surgical or transbronchial biopsy alone can be misleading, if not dangerous, since different ILDs, with distinct prognoses and treatment options, may have a similar pathological pattern (1,3,4).
Therefore, since the beginning of the 21st century, multidisciplinary discussion has become the “gold standard” approach for the diagnosis of ILDs, mainly IIPs. Robust communication and close collaboration amongst clinicians (primary care physicians, pulmonologists, and rheumatologists), radiologists, and pathologists is essential to reach the correct and final diagnosis. While in some cases, clinical and radiological information (from a high resolution chest computed tomogram) alone may lead to the diagnosis, cytological or histological information is warranted in others (Figure 2) (1-4,6).
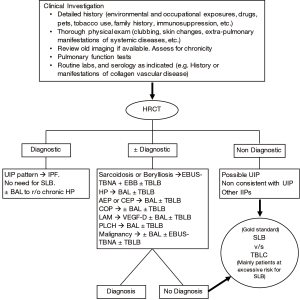
This article will review both the role as well as the limitations of bronchoscopy in the diagnosis of various ILDs. Since ILDs span a wide array of diseases, we shall limit our treatise to the more common ILDs, particularly the disorders where bronchoscopy plays a role in the diagnosis.
Bronchoscopy: a brief historical narrative
Bronchoscopy evolved continuously within the last century in order to offer a less invasive diagnostic and therapeutic approach to many lung diseases. This review is a brief report on the historical advancement of different bronchoscopic techniques used in diagnosing various ILDs.
The year 1897 witnessed the birth of bronchoscopy by the German otolaryngologist Gustav Killian, known today as the “father of bronchoscopy”. Killian performed a direct endoscopy on a volunteer, and examined the proximal airways for the first time in history. Later the same year, he proceeded to endoscopically remove the first foreign body (pork bone) from the main bronchus (7). He presented his new method “direct bronchoscopy” the following year at the Society of South German Laryngologists meeting, later publishing it (8). A few years later, in 1904, Chevalier Jackson, the “father of American bronchoesophagology”, introduced the first American illuminated “rigid bronchoscope” (9,10).
In 1962, Shigeto Ikeda, a Japanese thoracic surgeon, approached the Machida Corporation with the idea of developing a flexible bronchoscope with a diameter of less than 6 mm. After a few prototypes and revisions, the first functional flexible bronchoscope was unveiled at a meeting in Copenhagen in 1966. A few improvements later, including the adoption of a working channel, the Machida flexible bronchoscope became commercially available in 1968 (9-11). In 1970, the first Olympus model with better imaging and easy handling capabilities became commercially available (9).
After the introduction of flexible bronchoscopy, the usage of rigid scopes experienced a transient decline, until the early 1980’s when therapeutic uses (laser, stenting, etc.) gave it headway once again (10).In terms of interventions, bronchoalveolar lavage (BAL) began to be performed through the rigid bronchoscope in the early 20th century, mainly for the purpose of removing purulent secretions from the airways of patients with bronchiectasis. Cantrell et al. were the first to perform BALs in normal volunteers using the flexible scope in 1972 (12). Several studies followed, with further analysis of BAL cells and protein content, leading to an increase in the use of BAL in multiple lung diseases including some ILDs (13,14).
Transbronchial (transbronchoscopic) lung biopsy was first reported by Anderson et al. in 1965. He described this technique using a rigid bronchoscope in a small series of 13 patients (15). Subsequently, in 1972, Anderson published a larger case series of 450 patients undergoing transbronchial lung biopsy (TBLB) for DPLD (16). Shortly thereafter, TBLB was successfully performed through a flexible bronchoscope (17), leading to its widespread use until this day. The year 1978 saw the description of transbronchial needle aspiration by Wang et al., wherewith appropriate diagnostic tissue was obtained in 3 out of 5 patients (18).
Radial endobronchial ultrasound (EBUS) was introduced in the early 1990’s, and its usefulness was first reported by Hürter and Hanrath in a series of 100 patients (19). One major limitation for the radial probe was obtaining the biopsy blindly after localizing the lesion. In 2004, linear EBUS use was highlighted after Yasufuku and colleagues demonstrated the high diagnostic yield of the convex ultrasound probe in sampling mediastinal lesions, and distinguishing between malignant and benign lymph nodes (20). EBUS became the best first diagnostic tool to obtain tissue for non-small cell lung cancer diagnosis and staging (21). Furthermore, when compared to endobronchial and transbronchial biopsies, EBUS had a higher yield for the diagnosis of sarcoidosis (22).
As for cryotherapy, it has been in use since the 1970’s through rigid scopes, but it was not until 1996 when Mathur et al. described endobronchial cryotherapy with a flexible bronchoscope (23). Since about 2009, transbronchial cryobiopsy emerged as a new diagnostic tool for ILDs and mainly IIPs. As more data continue to emerge, transbronchial cryobiopsy may largely replace surgical lung biopsy (SLB) in becoming the first diagnostic modality to obtain tissue for IIPs diagnosis. We will review the advances to-date in cryobiopsy in a later paragraph.
The role of bronchoalveolar lavage
BAL is a relatively low risk technique performed during flexible bronchoscopy, allowing the recovery of cellular and non-cellular components from the epithelial surface of the alveoli and terminal bronchioles (14,24). Analysis of BAL cell counts, culture, and cytology provides valuable information that may lead to making a diagnosis or ruling out another (14,25). Standardization of BAL collection techniques and sample processing is critical especially when ILD is suspected (26,27). For many decades, BAL has been part of the workup of ILD, during which its role was mainly studied in patients with sarcoidosis, hypersensitivity pneumonitis (HP), and idiopathic pulmonary fibrosis (IPF) (14). Today, however, this role seems to be diminishing with the advancement of high resolution computed tomogram (HRCT) imaging of the chest. In the right clinical setting, HRCT findings may be diagnostic and a BAL might not be required (27). When a BAL is indicated in a patient with suspected ILD, it is preferable for it to be guided by a pre-procedural chest HRCT. A differential cell count should be performed in all patients, with cultures and cytology if clinically indicated. A lymphocyte subset analysis should not be routinely ordered (27).
In healthy volunteers, macrophages predominate as they represent at least 80% of nucleated white blood cells. Lymphocytes constitute 5–15%, neutrophils 2–3%, and eosinophils <1%. Any insult to the lungs leads to a perturbation of the epithelial layer at the level of the distal airways, and to a change in the cellular and non-cellular content of the BAL. By way of example, smoking increases the absolute number of BAL macrophages and neutrophils (27,28). A BAL cell differential count with >15% lymphocytes, >3% neutrophils, >1% eosinophils, or >0.5% mastocytes represents a BAL with lymphocytic, neutrophilic, eosinophilic, or mastocytic pattern respectively. An increase in more than one type of cells in the BAL represents a mixed cellular pattern. The presence of >5% epithelial cells in the sample suggests a suboptimal BAL, and cellular patterns should be interpreted with caution (27).
Various patterns, including lymphocytic, eosinophilic, neutrophilic, and mixed, can be found in patients with ILD, and the significance of each one varies significantly from one disease to another (Tables 1,2). The usefulness of such patterns is the subject of ongoing discussions, since, on one hand, most of these findings have low specificity and sensitivity, while on the other hand, studies that looked into the predictive value of BAL cell differentials in the diagnosis of ILD predate the era of wide use of HRCT (27,29,30).
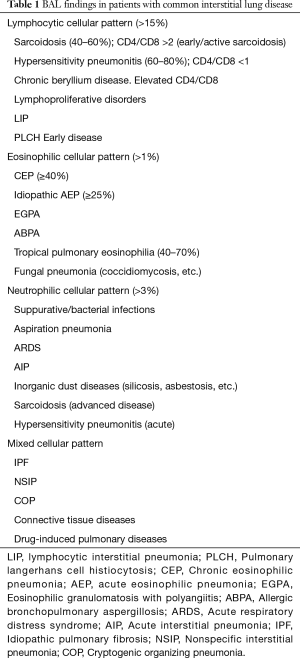
Full table

Full table
In most ILDs, BAL findings are classified as either nonspecific, consistent with or suggestive of a certain diagnosis. While BAL may not be diagnostic in and of itself, when combined with clinical and radiographic data, it might assist in reaching a diagnosis. It is only in a few situations that BAL findings could be highly diagnostic, even pathognomonic (27,31,32). BAL findings can be diagnostic in idiopathic acute eosinophilic pneumonias (33,34), chronic eosinophilic pneumonia (35), primary pulmonary lymphoma and lymphangitic carcinomatosis (36), pulmonary alveolar proteinosis (37), lipoid pneumonia (38,39), pulmonary Langerhans cell histiocytosis (PLCH) (40-42), and diffuse alveolar hemorrhage (43,44) (Tables 1,2).
BAL lymphocytosis is a sensitive, but nonspecific finding in sarcoidosis and HP (30,45). In fact, in the setting of an atypical HRCT, absence of BAL lymphocytosis may help in excluding HP and sarcoidosis (29). The CD4/CD8 ratio is usually decreased in HP (ratio <1), but increased in sarcoidosis (ratio >2). However, low and high ratios can be seen in sarcoidosis and HP respectively (45-48).
BAL has a limited role in the diagnosis of IPF. When performed, it serves mainly to exclude other processes in the setting of a non-diagnostic HRCT (27,29). In a study by Ohshimo et al., BAL lymphocytosis (>30%) was found in 6 out of 74 patients with a clinical and radiological diagnosis of IPF, and a different diagnosis was eventually made in all six cases (49). In almost a third of patients with HP, the responsible antigen may not be identifiable despite a thorough history (50). Hence, in patients with usual interstitial pneumonia (UIP) pattern on HRCT, a lymphocytic BAL (>40%) may suggest HP, and lead to further investigate possible environmental exposures or even perform a lung biopsy (2). In patients with IPF exacerbation, BAL has a disputed role, and is not always required to exclude infections (51).
BAL has no defined role in patients with non-specific interstitial pneumonia (NSIP), where it is reported to be lymphocytic in most, but not all studies. In one study comparing BAL cell counts among patients with IPF and patients with fibrotic NSIP, no difference was seen, and both groups had a non-lymphocytic BAL (52). In patients with cryptogenic organizing pneumonia (COP), BAL has a nonspecific mixed cellular pattern, and is helpful only to exclude other diagnoses (53,54). In patients with respiratory bronchiolitis interstitial lung disease (RBILD) or desquamative interstitial pneumonia (DIP), BAL has an excess of pigmented macrophages without other abnormalities, similarly to a healthy smoker (55). Absence of BAL lymphocytosis may be occasionally diagnostic of RBILD when combined with a HRCT suggestive of either HP or RBILD (29).
BAL cell counts are highly variable in connective tissue disease related ILDs, and have no diagnostic value (56). Since drugs have been linked to various types of lung injuries or ILDs (i.e., HP, eosinophilic pneumonia, organizing pneumonia, diffuse alveolar hemorrhage, etc.), BAL findings are usually heterogeneous, but may have some correlation with the type of lung injury caused by the culprit drug (57). In the right clinical setting, BAL may have some value in suggesting a drug related lung injury, but it is mainly performed to exclude infection or malignancy (36,57).
Many researchers looked into the benefit of BAL in monitoring disease activity in patients with ILD, but so far no clinical applicability exists. Some studies found that in the same disease, different BAL patterns correlated with different disease stages, likelihood of progression, and prognosis (i.e., in patients with presumed IPF, a lymphocytic BAL correlated with better prognosis) (58-61). The major caveat however is that most of these studies are dated, thus increasing the likelihood of the included patients having other IIPs. UIP and NSIP for example may coexist in the same patient, and prognosis is usually driven by the disease with the worst prognosis (59). Although BAL might not have a role in monitoring disease activity, it might serve to rule out infections and complications related to ILD treatment (drug toxicities, etc.).
The role of endobronchial biopsy (EBB)
The role of EBB in the workup of patients with ILDs has so far been relatively small. It mainly serves to diagnose sarcoidosis and chronic beryllium disease (CBD). Four to six endobronchial biopsies are usually obtained, preferably from a site where the mucosa is abnormal or from the first and second carina if the mucosa appears normal: 30% of patients with normal mucosa may have positive EBB (62). One study revealed that positive EBB correlates with a more aggressive disease (63). When supplementing transbronchial biopsy, EBB was found to increase the diagnostic yield of sarcoidosis by 10–20% (62,64). Nearly 6% of all patients diagnosed with sarcoidosis actually have CBD. A history of Beryllium exposure should prompt a diagnostic evaluation for CBD (65).
The role of transbronchial biopsy
Nowadays, TBLB is mostly performed using the flexible bronchoscope, and with some form of guidance (fluoroscopy, ultrasound, or electromagnetic navigation). Although the introduction of guidance improved the diagnostic yield of TBLB, it is unclear whether it added to its safety. TBLB related mortality is rare (<0.05%), while bleeding (1–4%) and pneumothorax (1–6%) constitute the main complications (3,64,66-69). Bilateral TBLBs should not be performed during the same procedure under any circumstance, because of the risk of bilateral iatrogenic pneumothoraces. The optimal number of TBLB is unknown, but in general, four to six biopsy specimens should suffice for most patients with diffuse lung diseases (3,70-73). It is unlikely that the alligator forceps has a better diagnostic yield compared to the cup forceps. Nonetheless, specimens obtained are larger and complication rates are lower with the former (64,70,74).
The overall diagnostic yield of TBLB is around 25–75%, but it varies largely depending on the underlying ILD (3,73,75,76). It can be as low as 20–30% for IPF (77,78), and as high as 80–90% for non-fibrotic ILDs (69,73,78,79). The small size of the biopsy specimen (median size 5mm), and the high probability of crush artifacts, limit the role of TBLB in the workup of several ILDs (3,80).However, TBLB continues to be the procedure of choice when the suspected disease has a peribronchovascular or centrilobular involvement, and when small samples are enough to establish a certain diagnosis (3,81,82).
TBLB can be diagnostic in sarcoidosis, CBD, HP, eosinophilic pneumonia, PLCH, COP, lymphangitic carcinomatosis, diffuse alveolar damage, amyloidosis, PAP, alveolar microlithiasis, and multiple infections (3,53,76,82-89).
The diagnostic yield of TBLB for sarcoidosis varies from one stage to another., with a higher yield observed in patients with stage II and III disease (66–95% in comparison to 12–55% in stage I disease) (64,73,90-92). EBB and TBNA (conventional or ultrasound guided), when added to TBLB, increase the overall diagnostic yield of the procedure (62,64,92,93). Oftentimes, a TBLB is not needed to diagnose HP since the diagnosis may be made with confidence based on clinical and radiological information (94-96). However, in few cases, histological diagnosis may be needed, and TBLB should be considered first (3,73). Yet, in chronic HP, TBLB is unlikely to be diagnostic and SLB may be preferred (97).
While clinical features and HRCT findings may be suggestive of PLCH in some patients, confirmation of the diagnosis by BAL or biopsy is generally recommended (86). In a recent case series of patients with PLCH, TBLB specimens were diagnostic in 50% (19/38) of the patients (41). TBLB has a yield of approximately 60% in patients with lymphangioleiomyomatosis (87). However, per the 2016 American Thoracic Society/Japanese Respiratory Society guidelines, the diagnosis of LAM can be usually reached based on clinical features and HRCT findings. Even when typical clinical features are absent, testing for vascular endothelial growth factor-D (VEGF-D) is recommended first, since a high level (>800 pg/mL) may obviate the need for biopsy in almost 70% of patients (98). A confident diagnosis of PAP can usually be made based on history, radiological findings (HRCT), BAL appearance and cytology, and compatible biomarkers (85). In a large cohort of patients with PAP, TBLB was done only in a third of patients (99).
In patients with suspected COP, TBLB should be considered first (prior to more invasive procedures like video-assisted thoracoscopic surgery), since, in many instances, it may be sufficient to make a confident diagnosis (3,54,100). In a prospective analysis of patients with clinical and radiological features of COP, the sensitivity and specificity of TBLB were 64% and 86% respectively (101).
The diagnosis of IPF requires exclusion of other known causes of ILD, and the presence of either a UIP pattern on HRCT (subpleural and predominantly basal distribution of reticular abnormalities and honeycombing, with absence of features listed as inconsistent with UIP), or specific combinations of HRCT and SLB. The latest guidelines pertinent to the diagnosis and management of IPF included a weak recommendation with low-quality evidence against using TBLB in the evaluation of IPF in the majority of patients (2). In a retrospective analysis of patients with proven UIP, TBLB specimens were diagnostic of UIP (honeycombing, patchy fibrosis, and fibroblastic foci) in 7 out of 22 patients (79). In another series by Tomassetti et al., TBLB was found to detect UIP in 30% of the cases, with high specificity and positive predictive value, but low negative predictive value (78). Sheth et al. recently noted that TBLB combined with clinical and HRCT data, provided enough information to make a confident and accurate diagnosis in 20–30% of patients with fibrotic ILD (77). Despite its poor sensitivity, TBLB might be a reasonable option for patients with suspected fibrotic ILD, who cannot tolerate SLB (or transbronchial cryobiopsy). Prospective studies investigating the definite role of TBLB in IIPs are yet to come.
The role of cryobiopsy
Surgical lung biopsies (SLB) have for years been the gold standard for diagnosis of IIPs, in particular when a non-invasive diagnosis cannot be made with confidence (2,4). Owing mainly to a significantly larger size of tissue sample, SLB was favored over traditional transbronchial forceps biopsy, with less crush artifact and sampling error. The limiting factor had always been the health status of the patient, alongside the high risk of morbidity and mortality associated with the surgical approach. IPF stands out as the prototype of such complications, ever since early data placed the mortality risk as high as 21.7% (102). A safe, high-yield, less-invasive and more effective alternative was thus desired. While cryotherapy dates back to the 19th century when it was used in a limited manner for control of local pain, the use of liquefied gases helped propel it forward. In 1961, Cooper and Lee introduced cryostatic congelation in treating certain neurological conditions (103). Since that time, many advances occurred, eventually resulting in the use of cryotherapy in pulmonary medicine, through the application of rapid freeze-thaw cycles. For several years, cryotherapy was employed for the purpose of treating central airway obstruction (of benign or malignant etiology) (23), early superficial tumors (104), removal of foreign bodies (105), control of bleeding, or removal of granulation tissue (which might form inside an endobronchial stent or at sites of anastomoses) (106).
In 2009, Babiak introduced the use of a cryoprobe for the purpose of obtaining lung biopsies during flexible bronchoscopy (80). In comparing traditional forceps transbronchial biopsies to cryobiopsy performed successively in 41 patients with DPLDs, he found that the mean specimen area in the latter was nearly three times larger than that in the former (15.11 vs. 5.82 mm2; P<0.01). Only two patients developed a pneumothorax, while bleeding did not require any intervention. Since then, there has been a surge of data on the use of this technique for the diagnosis of parenchymal lung disease. From a technical standpoint, the procedure requires the use of a cryoprobe (rigid, semi-rigid or flexible), bronchoscope, and a cryogen (cooling agent). Flexible cryoprobes exist in three diameter sizes (2.4, 1.9 and 1.1 mm), and various lengths ranging from 780 to 1,050 mm. The most commonly used is the Erbe 1.9 mm diameter flexible cryoprobe (Erbe Elektromedizin GmBH, Germany). Nitrous oxide (lowest temperature −89 °C), liquid nitrogen (−196 °C) or carbon dioxide (−79.3 °C) may be used as cryogens. The use of liquid nitrogen in the USA is limited by lack of availability, difficulty in handling and greater tissue damage. On the other hand, CO2 may cause snow during bronchoscopy. Therefore nitrous oxides the most commonly used gas. The cryotherapy system uses a Joule-Thompson effect where the pressurized gas is released at high flow from the tip of the probe, and rapidly expands at atmospheric pressure, causing a large decrease in temperature, which leads the two most distal centimeters of the probe to freeze the surrounding tissue and adhere to it, easily detaching it. The probes are reusable, and the technology is relatively inexpensive (107).
In a recent systematic review of 11 studies (731 patients), the diagnostic yield ranged from 51% to 98% (pooled estimate 83%; 95% CI, 73–94) when the biopsy was reviewed in a multidisciplinary setting (108). Furthermore, prospective data revealed that the diagnostic yield for DPLD increased from 69% to96% when biopsies were obtained from two—rather than one—segments of the same lobe (109). Moreover, retrospective data indicates that transbronchial lung cryobiopsy (TBLC) improves the yield of transbronchial biopsy when performed sequentially and reviewed using a multidisciplinary approach (110).
Early reports of TBLC were concerning for a high risk of both bleeding and pneumothorax. In one of the largest systematic reviews to-date, the frequency of moderate to severe bleeding ranged from 0% to 78% (pooled estimate 39%; 95% CI, 3–76), while that of pneumothorax ranged from 0% to 24.9% (pooled estimate 12%; 95% CI, 3–21) (107,111). In a retrospective analysis of 447 cases with ILD that could not be diagnosed noninvasively (possible UIP or inconsistent with UIP), 150 patients underwent SLB and 297 underwent TBLC (111). Pneumothorax was the most common complication in TBLC (20.2%). Median time of hospitalization was significantly less in TBLC (2.6 vs. 6.1 days; P<0.0001), and so was mortality due to adverse events (0.3% vs. 2.7%; P=0.045). No severe bleeding was observed. TBLC was diagnostic for 82.8% of patients and SLB for 98.7% (P=0.013). The same group performed a meta-analysis of 15 investigations (781 patients) which revealed a diagnostic yield of 0.81 (0.75–0.87). In the same article, the pooled probability of developing a pneumothorax (994 patients) was 0.06 (95% CI, 0.02–0.11), while that of bleeding (383 patients) was 0.12 (95% CI, 0.02–0.25).
In a recent report, DiBardino et al. described a series of 25 consecutive TBLC marred with a high rate of complications: 6 patients (24%), including 3 (12%) who suffered severe bleeding, 2 (8%) an iatrogenic pneumothorax and 1 (4%) hypercapnic respiratory failure (112). However, the authors performed TBLC admittedly without a strict protocol. Twenty-one patients underwent the procedure via a laryngeal mask airway which could limit bleeding control, while fluoroscopy was used in only 10 cases which could limit visualization of the probe in respect to the pleura. This report rather indicates the need for adequate planning prior to performing TBLC and anticipation of complications, thus highlighting the need to be proactive in implementing an adequate protocol and the caution to employ proper safeguards.
In an interim analysis of an ongoing prospective trial, SLB was deemed unnecessary following TBLC (113). Bleeding rate was 78%, pneumothorax 22%, while 30-day mortality was 0.7% when compared to SLB (3.3%). The prophylactic deployment of a Fogarty balloon (or a bronchial blocker) after retracting the cryobiopsy sample may prevent severe hemorrhage, a technical approach growing in popularity.
In most reports, and on average, cryobiopsy sample size varies in diameter from 9.2 mm (range, 2–20 mm; SD 3.9) (114) to 14.7 (°¿11 mm) (115). The median number of samples varies by reports (typically from two to six), but is usually less than that of traditional forceps biopsies, with larger sample size and higher yield (109). In a perspectives pathology article, a review of various studies showed that TBLC offers a mean specimen diameter between 4 and 9 mm, and an area between 9 and 64.2 mm2. The same study reported yields ranging from 70% to 95%, with a risk of pneumothorax ranging from 2.6% to 33%, and that of significant bleeding from 1.4% to 56%. Although they found the emerging data encouraging, the pathologists concluded that further study is needed before determining whether TBLC is en route to replace SLB (116).
Cryobiopsy has been shown to be sufficient for the diagnosis of UIP (117), NSIP (110), constrictive bronchiolitis (118), desquamative interstitial pneumonia (119), HP (111), RBILD (110), organizing pneumonia (108), diffuse alveolar damage (108), sarcoidosis (109), drug toxicity (109) and others (eosinophilic pneumonia, PAP, amyloidosis, follicular bronchiolitis, PLCH) (108,111). The individual yield for each entity remains to be determined.
Therefore, when compared to traditional SLB, transbronchial cryobiopsy offers a comparable—and at times higher—yield (particularly in the setting of a multidisciplinary discussion), with a shorter hospitalization duration, a lesser risk of adverse events and mortality from such events, at a lower cost (120). When compared to forceps biopsy, it offers larger specimens, a higher diagnostic yield and less artifact (109,121). The most likely benefit appears to be a decreased need for SLB to diagnose IPF, and the added assurance to a multidisciplinary approach to diagnosis (122). There cent development of a novel sheath cryobiopsy may prevent the need to remove the bronchoscope in its entirety after each specimen retrieval (123), a technique that remains to be vindicated in-vivo (124). Since most of the currently available data is retrospective in nature, and the standardization process is still underway, the role of TBLC in the diagnosis of ILD remains subject to expansion and significant future development, even as technical advances might limit the frequency of complications, mainly hemorrhage and pneumothorax. For the time being, given lack of standardization of cryobiopsy technique, the variable rate of complications and yield, it seems prudent that TBLC be performed by experienced bronchoscopists at a high volume center with a clear protocol, competent staff and advanced preparedness for possible complications and the remedy thereof.
The role of endobronchial ultrasound
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) typically serves to evaluate enlarged mediastinal and hilar lymph nodes (20). It is mainly used in the diagnosis, staging, and restaging of patients with suspected or known non-small cell lung cancer (21). The clinical utility of EBUS-TBNA in the diagnosis of lymphoma remains controversial despite recent data suggesting high sensitivity (89%) and specificity (97%) (125). In sarcoidosis, conventional or EBUS-guided TBNA has a great diagnostic value, especially when combined with endobronchial and transbronchial biopsies (22,92,126-128). When compared to standard TBNA, EBUS-TBNA had a better yield in the diagnosis of sarcoidosis (129). Moreover, EBUS-TBNA with rapid on site evaluation may obviate the need to perform unnecessary TBB, while maintaining a high diagnostic yield for sarcoidosis (130). EBUS-TBNA may play a minor role as well in the diagnosis of fungal and mycobacterial infections (129,131). Endobronchial ultrasound using the radial probe has no defined role yet in the workup of ILD. Only recently, a small case series suggested that the use of radial ultrasound to guide transbronchial cryobiopsy may lead to a decrease in the risk of major bleeding (132).
Conclusions
ILDs remain some of the most challenging medical conditions, both to diagnose and to treat. A multidisciplinary approach carries the highest promise, particularly when coupled with proper pathology. Without a correct diagnosis, prognosis may be quite inaccurate, and curative therapy might not be administered. Therefore, differentiating between the various ILDs is paramount and ever warranted. And bronchoscopy remains one of the less invasive and more prime approaches for such an end.
As we have seen, bronchoscopic techniques emerged and progressed in the span of a few decades. Their growth has been exponential, their safety has been improving, and their use has been expanding. While their role in diagnosing ILDs varies from one technique to another, and one disease to the next, the latest advances in cryobiopsy appear to carry great promise. With early reports raising concerns for high rates of pneumothorax and bleeding, the improving performance and increasing yield compared to transbronchial and SLB in the span of a few years, might herald a safe, less costly and higher yield alternative for the diagnosis of ILDs—even fibrosing types—in our own lifetime. If the recent past has carried such excitement, the future can only hold promises.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63 Suppl 5:v1-58. [Crossref] [PubMed]
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Society BT, Committee SO. The Diagnosis, Assessment and Treatment of Diffuse Parenchymal Lung Disease in Adults. Thorax 1999;54:S1-28. [Crossref] [PubMed]
- Raghu G. Interstitial lung disease: a diagnostic approach. Are CT scan and lung biopsy indicated in every patient? Am J Respir Crit Care Med 1995;151:909-14. [Crossref] [PubMed]
- Kollofrath O. Entfernung eines Knochenstücks aus dem rechten Bronchus auf natürlichem Wege und unter Anwendung der directen Laryngoscopie. MMW 1897;38:1038-9.
- Killian G. Ueber directe bronchoscopie. MMW 1898;27:844-7.
- Ernst A. Introduction to bronchoscopy. Cambridge: Cambridge University Press, 2009.
- Panchabhai TS, Mehta AC. Historical perspectives of bronchoscopy. Connecting the dots. Ann Am Thorac Soc 2015;12:631-41. [Crossref] [PubMed]
- Ikeda S, Yanai N, Ishikawa S. Flexible bronchofiberscope. Keio J Med 1968;17:1-16. [Crossref] [PubMed]
- Cantrell ET, Martin RR, Warr GA, et al. Induction of aryl hydrocarbon hydroxylase in human pulmonary alveolar macrophages by cigarette smoking. Trans Assoc Am Physicians 1973;86:121-30. [PubMed]
- Reynolds HY, Newball HH. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med 1974;84:559-73. [PubMed]
- Reynolds HY. Bronchoalveolar Lavage 1, 2. American Review of Respiratory Disease. Am Thoracic Soc 1987;135:250-63.
- Andersen HA, Harrison EG. Transbronchoscopic lung biopsy in diffuse pulmonary disease. Trans Annu Meet Am Bronchoesophagol Assoc 1965;45:84-91. [PubMed]
- Andersen HA, Fontana RS. Transbronchoscopic lung biopsy for diffuse pulmonary diseases: technique and results in 450 cases. Chest 1972;62:125-8. [Crossref] [PubMed]
- Levin DC, Wicks AB, Ellis JH. Transbronchial lung biopsy via the fiberoptic bronchoscope. Am Rev Respir Dis 1974;110:4-12. [PubMed]
- Wang KP, Terry P, Marsh B. Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am Rev Respir Dis 1978;118:17-21. [PubMed]
- Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992;47:565-7. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- Von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013;309:2457-64. [Crossref] [PubMed]
- Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest 1996;110:718-23. [Crossref] [PubMed]
- Clinical guidelines and indications for bronchoalveolar lavage (BAL): Report of the European Society of Pneumology Task Group on BAL. Eur Respir J 1990;3:937-76. [PubMed]
- Goldstein RA, Rohatgi PK, Bergofsky EH, et al. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis 1990;142:481-6. [Crossref] [PubMed]
- Haslam PL, Baughman RP. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J 1999;14:245-8. [Crossref] [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. The BAL Cooperative Group Steering Committee. Am Rev Respir Dis 1990;141:S169-202. [PubMed]
- Wells AU. The clinical utility of bronchoalveolar lavage in diffuse parenchymal lung disease. Eur Respir Rev 2010;19:237-41. [Crossref] [PubMed]
- Welker L, Jörres RA, Costabel U, et al. Predictive value of BAL cell differentials in the diagnosis of interstitial lung diseases. Eur Respir J 2004;24:1000-6. [Crossref] [PubMed]
- Meyer KC. The role of bronchoalveolar lavage in interstitial lung disease. Clin Chest Med 2004;25:637-49. [Crossref] [PubMed]
- Kilinç G, Kolsuk EA. The role of bronchoalveolar lavage in diffuse parenchymal lung diseases. Curr Opin Pulm Med 2005;11:417-21. [Crossref] [PubMed]
- Philit F, Etienne-Mastroïanni B, Parrot A, et al. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med 2002;166:1235-9. [Crossref] [PubMed]
- Allen J. Acute eosinophilic pneumonia. Semin Respir Crit Care Med 2006;27:142-7. [Crossref] [PubMed]
- Marchand E, Cordier JF. Idiopathic chronic eosinophilic pneumonia. Orphanet J Rare Dis 2006;1:11. [Crossref] [PubMed]
- Rennard SI. Bronchoalveolar lavage in the diagnosis of cancer. Lung 1990;168 Suppl:1035-40. [Crossref] [PubMed]
- Chou CW, Lin FC, Tung SM, et al. Diagnosis of pulmonary alveolar proteinosis: usefulness of papanicolaou-stained smears of bronchoalveolar lavage fluid. Arch Intern Med 2001;161:562-6. [Crossref] [PubMed]
- Lauque D, Dongay G, Levade T, et al. Bronchoalveolar lavage in liquid paraffin pneumonitis. Chest 1990;98:1149-55. [Crossref] [PubMed]
- Corwin RW, Irwin RS. The lipid-laden alveolar macrophage as a marker of aspiration in parenchymal lung disease. Am Rev Respir Dis 1985;132:576-81. [PubMed]
- Auerswald U, Barth J, Magnussen H. Value of CD-1-positive cells in bronchoalveolar lavage fluid for the diagnosis of pulmonary histiocytosis X. Lung 1991;169:305-9. [Crossref] [PubMed]
- Baqir M, Vassallo R, Maldonado F, et al. Utility of bronchoscopy in pulmonary Langerhans cell histiocytosis. J Bronchology Interv Pulmonol 2013;20:309-12. [Crossref] [PubMed]
- Takizawa Y, Taniuchi N, Ghazizadeh M, et al. Bronchoalveolar lavage fluid analysis provides diagnostic information on pulmonary Langerhans cell histiocytosis. J Nippon Med Sch 2009;76:84-92. [Crossref] [PubMed]
- Grebski E, Hess T, Hold G, et al. Diagnostic value of hemosiderin-containing macrophages in bronchoalveolar lavage. Chest 1992;102:1794-9. [Crossref] [PubMed]
- Ioachimescu OC, Stoller JK. Diffuse alveolar hemorrhage: diagnosing it and finding the cause. Cleve Clin J Med 2008;75:258-260, 264-5 passim. [Crossref] [PubMed]
- Winterbauer RH, Lammert J, Selland M, et al. Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest 1993;104:352-61. [Crossref] [PubMed]
- Kantrow SP, Meyer KC, Kidd P, et al. The CD4/CD8 ratio in BAL fluid is highly variable in sarcoidosis. Eur Respir J 1997;10:2716-21. [Crossref] [PubMed]
- Barrera L, Mendoza F, Zuñiga J, et al. Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med 2008;177:44-55. [Crossref] [PubMed]
- Verstraeten A, Demedts M, Verwilghen J, et al. Predictive value of bronchoalveolar lavage in pulmonary sarcoidosis. Chest 1990;98:560-7. [Crossref] [PubMed]
- Ohshimo S, Bonella F, Cui A, et al. Significance of bronchoalveolar lavage for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:1043-7. [Crossref] [PubMed]
- Vourlekis JS, Schwarz MI, Cherniack RM, et al. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med 2004;116:662-8. [Crossref] [PubMed]
- Petrosyan F, Culver DA, Reddy AJ. Role of bronchoalveolar lavage in the diagnosis of acute exacerbations of idiopathic pulmonary fibrosis: a retrospective study. BMC Pulm Med 2015;15:70. [Crossref] [PubMed]
- Veeraraghavan S, Latsi PI, Wells AU, et al. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur Respir J 2003;22:239-44. [Crossref] [PubMed]
- Cottin V, Cordier JF. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med 2012;33:462-75. [Crossref] [PubMed]
- Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006;28:422-46. [Crossref] [PubMed]
- Ryu JH, Colby TV, Hartman TE, et al. Smoking-related interstitial lung diseases: a concise review. Eur Respir J 2001;17:122-32. [Crossref] [PubMed]
- Wallaert B, Rossi GA, Sibille Y. Clinical guidelines and indications for bronchoalveolar lavage (BAL): collagen-vascular diseases. Eur Respir J 1990;3:942-3, 961-9. [PubMed]
- Costabel U, Uzaslan E, Guzman J. Bronchoalveolar lavage in drug-induced lung disease. Clin Chest Med 2004;25:25-35. [Crossref] [PubMed]
- Drent M, van Velzen-Blad H, Diamant M, et al. Bronchoalveolar lavage in extrinsic allergic alveolitis: effect of time elapsed since antigen exposure. Eur Respir J 1993;6:1276-81. [PubMed]
- Watters LC, Schwarz MI, Cherniack RM, et al. Idiopathic pulmonary fibrosis. Pretreatment bronchoalveolar lavage cellular constituents and their relationships with lung histopathology and clinical response to therapy. Am Rev Respir Dis 1987;135:696-704. [PubMed]
- Kinder BW, Brown KK, Schwarz MI, et al. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 2008;133:226-32. [Crossref] [PubMed]
- Poulter LW, Rossi GA, Bjermer L, et al. The value of bronchoalveolar lavage in the diagnosis and prognosis of sarcoidosis. Eur Respir J 1990;3:943-4. [PubMed]
- Shorr AF. Endobronchial Biopsy for Sarcoidosis. Chest 2001;120:109-14. [Crossref] [PubMed]
- Bjermer L, Thunell M, Rosenhall L, et al. Endobronchial biopsy positive sarcoidosis: relation to bronchoalveolar lavage and course of disease. Respir Med 1991;85:229-34. [Crossref] [PubMed]
- Sehgal IS, Bal A, Dhooria S, et al. A Prospective Randomized Controlled Trial Comparing the Efficacy and Safety of Cup vs Alligator Forceps for Performing Transbronchial Lung Biopsy in Patients With Sarcoidosis. Chest 2016;149:1584-6. [Crossref] [PubMed]
- Balmes JR, Abraham JL, Dweik RA, et al. An official American Thoracic Society statement: diagnosis and management of beryllium sensitivity and chronic beryllium disease. American journal of respiratory and critical care medicine. Am J Respir Crit Care Med 2014;190:e34-59. [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest 1995;107:430-2. [Crossref] [PubMed]
- Sindhwani G, Shirazi N, Sodhi R, et al. Transbronchial lung biopsy in patients with diffuse parenchymal lung disease without 'idiopathic pulmonary fibrosis pattern' on HRCT scan - Experience from a tertiary care center of North India. Lung India 2015;32:453-6. [Crossref] [PubMed]
- Curley FJ, Johal JS, Burke ME, et al. Transbronchial lung biopsy: can specimen quality be predicted at the time of biopsy? Chest 1998;113:1037-41. [Crossref] [PubMed]
- de Boer S, Milne DG, Zeng I, et al. Does CT scanning predict the likelihood of a positive transbronchial biopsy in sarcoidosis? Thorax 2009;64:436-9. [Crossref] [PubMed]
- Roethe RA, Fuller PB, Byrd RB, et al. Transbronchoscopic lung biopsy in sarcoidosis. Optimal number and sites for diagnosis. Chest 1980;77:400-2. [Crossref] [PubMed]
- Descombes E, Gardiol D, Leuenberger P. Transbronchial lung biopsy: an analysis of 530 cases with reference to the number of samples. Monaldi Arch Chest Dis 1997;52:324-9. [PubMed]
- Jabbardarjani H, Eslaminejad A, Mohammadtaheri Z, et al. The effect of cup versus alligator forceps on the results of transbronchial lung biopsy. J Bronchology Interv Pulmonol 2010;17:117-21. [Crossref] [PubMed]
- MacJannette R, Fiddes J, Kerr K, et al. Is bronchoscopic lung biopsy helpful in the management of patients with diffuse lung disease? Eur Respir J 2007;29:1064. [Crossref] [PubMed]
- Ensminger SA, Prakash UB. Is bronchoscopic lung biopsy helpful in the management of patients with diffuse lung disease? Eur Respir J 2006;28:1081-4. [Crossref] [PubMed]
- Sheth JS, Belperio JA, Fishbein MC, et al. Utility of Transbronchial vs Surgical Lung Biopsy in the Diagnosis of Suspected Fibrotic Interstitial Lung Disease. Chest 2017;151:389-99. [Crossref] [PubMed]
- Tomassetti S, Cavazza A, Colby TV, et al. Transbronchial biopsy is useful in predicting UIP pattern. Respir Res 2012;13:96. [Crossref] [PubMed]
- Berbescu EA, Katzenstein AL, Snow JL, et al. Transbronchial biopsy in usual interstitial pneumonia. Chest 2006;129:1126-31. [Crossref] [PubMed]
- Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009;78:203-8. [Crossref] [PubMed]
- Poletti V, Chilosi M, Olivieri D. Diagnostic invasive procedures in diffuse infiltrative lung diseases. Respiration 2004;71:107-19. [Crossref] [PubMed]
- Leslie KO, Gruden JF, Parish JM, et al. Transbronchial biopsy interpretation in the patient with diffuse parenchymal lung disease. Arch Pathol Lab Med 2007;131:407-23. [PubMed]
- Raj R, Raparia K, Lynch DA, et al. Surgical Lung Biopsy for Interstitial Lung Diseases. Chest 2017;151:1131-40. [Crossref] [PubMed]
- Travis WD, Hunninghake G, King TE Jr, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008;177:1338-47. [Crossref] [PubMed]
- Suzuki T, Trapnell BC. Pulmonary Alveolar Proteinosis Syndrome. Clin Chest Med 2016;37:431-40. [Crossref] [PubMed]
- Tazi A. Adult pulmonary Langerhans' cell histiocytosis. Eur Respir J 2006;27:1272-85. [Crossref] [PubMed]
- Meraj R, Wikenheiser-Brokamp KA, Young LR, et al. Utility of transbronchial biopsy in the diagnosis of lymphangioleiomyomatosis. Front Med 2012;6:395-405. [Crossref] [PubMed]
- Kim CH, Kim S, Kwon OJ, et al. Pulmonary diffuse alveolar septal amyloidosis--diagnosed by transbronchial lung biopsy. Korean J Intern Med 1990;5:63-8. [Crossref] [PubMed]
- Cale WF, Petsonk EL, Boyd CB. Transbronchial biopsy of pulmonary alveolar microlithiasis. Arch Intern Med 1983;143:358-9. [Crossref] [PubMed]
- Gilman MJ, Wang KP. Transbronchial lung biopsy in sarcoidosis. An approach to determine the optimal number of biopsies. Am Rev Respir Dis 1980;122:721-4. [Crossref] [PubMed]
- Koerner SK, Sakowitz AJ, Appelman RI, et al. Transbronchinal lung biopsy for the diagnosis of sarcoidosis. N Engl J Med 1975;293:268-70. [Crossref] [PubMed]
- Navani N, Booth HL, Kocjan G, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology 2011;16:467-72. [Crossref] [PubMed]
- Trisolini R, Lazzari Agli L, Cancellieri A, et al. The value of flexible transbronchial needle aspiration in the diagnosis of stage I sarcoidosis. Chest 2003;124:2126-30. [Crossref] [PubMed]
- Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med 2003;168:952-8. [Crossref] [PubMed]
- Johannson KA, Elicker BM, Vittinghoff E, et al. A diagnostic model for chronic hypersensitivity pneumonitis. Thorax 2016;71:951-4. [Crossref] [PubMed]
- Schuyler M, Cormier Y. The diagnosis of hypersensitivity pneumonitis. Chest 1997;111:534-6. [Crossref] [PubMed]
- Trahan S, Hanak V, Ryu JH, et al. Role of surgical lung biopsy in separating chronic hypersensitivity pneumonia from usual interstitial pneumonia/idiopathic pulmonary fibrosis: analysis of 31 biopsies from 15 patients. Chest 2008;134:126-32. [Crossref] [PubMed]
- McCormack FX, Gupta N, Finlay GR, et al. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am J Respir Crit Care Med 2016;194:748-61. [Crossref] [PubMed]
- Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008;177:752-62. [Crossref] [PubMed]
- Dina R, Sheppard MN. The histological diagnosis of clinically documented cases of cryptogenic organizing pneumonia: diagnostic features in transbronchial biopsies. Histopathology 1993;23:541-5. [Crossref] [PubMed]
- Poletti V, Cazzato S, Minicuci N, et al. The diagnostic value of bronchoalveolar lavage and transbronchial lung biopsy in cryptogenic organizing pneumonia. Eur Respir J 1996;9:2513-6. [Crossref] [PubMed]
- Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175-9. [Crossref] [PubMed]
- Cooper IS, Lee AS. Cryostatic congelation: a system for producing a limited, controlled region of cooling or freezing of biologic tissues. J Nerv Ment Dis 1961;133:259-63. [Crossref] [PubMed]
- Deygas N, Froudarakis M, Ozenne G, et al. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26-31. [Crossref] [PubMed]
- Reddy AJ, Govert JA, Sporn TA, et al. Broncholith removal using cryotherapy during flexible bronchoscopy: a case report. Chest 2007;132:1661-3. [Crossref] [PubMed]
- Maiwand MO, Zehr KJ, Dyke CM, et al. The role of cryotherapy for airway complications after lung and heart-lung transplantation. Eur J Cardiothorac Surg 1997;12:549-54. [Crossref] [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [Crossref] [PubMed]
- Johannson KA, Marcoux VS, Ronksley PE, et al. Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease. A Systematic Review and Metaanalysis. Ann Am Thorac Soc 2016;13:1828-38. [PubMed]
- Ravaglia C, Wells AU, Tomassetti S, et al. Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Disease: Comparison between Biopsy from 1 Segment and Biopsy from 2 Segments-Diagnostic Yield and Complications. Respiration 2017;93:285-92. [Crossref] [PubMed]
- Ramaswamy A, Homer R, Killam J, et al. Comparison of Transbronchial and Cryobiopsies in Evaluation of Diffuse Parenchymal Lung Disease. J Bronchology Interv Pulmonol 2016;23:14-21. [Crossref] [PubMed]
- Ravaglia C, Bonifazi M, Wells AU, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration 2016;91:215-27. [Crossref] [PubMed]
- DiBardino DM, Haas AR, Lanfranco AR, et al. High Complication Rate after Introduction of Transbronchial Cryobiopsy into Clinical Practice at an Academic Medical Center. Ann Am Thorac Soc 2017;14:851-857. [Crossref] [PubMed]
- Hagmeyer L, Theegarten D, Treml M, et al. Validation of transbronchial cryobiopsy in interstitial lung disease - interim analysis of a prospective trial and critical review of the literature. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:2-9. [PubMed]
- Ussavarungsi K, Kern RM, Roden AC, et al. Transbronchial cryobiopsy in diffuse parenchymal lung disease: retrospective analysis of 74 cases. Chest 2017;151:400-8. [Crossref] [PubMed]
- Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology 2014;19:900-6. [Crossref] [PubMed]
- Raparia K, Aisner DL, Allen TC, et al. Transbronchial Lung Cryobiopsy for Interstitial Lung Disease Diagnosis: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Poletti V, Ravaglia C, Gurioli C, et al. Invasive diagnostic techniques in idiopathic interstitial pneumonias. Respirology 2016;21:44-50. [Crossref] [PubMed]
- Lentz RJ, Fessel JP, Johnson JE, et al. Transbronchial Cryobiopsy Can Diagnose Constrictive Bronchiolitis in Veterans of Recent Conflicts in the Middle East. American journal of respiratory and critical care medicine. Am J Respir Crit Care Med 2016;193:806-8. [Crossref] [PubMed]
- Dias C, Mota P, Neves I, et al. Transbronchial cryobiopsy in the diagnosis of desquamative interstitial pneumonia. Revista portuguesa de pneumologia. Rev Port Pneumol 2006;2016:288-90.
- Sharp C, McCabe M, Adamali H, et al. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease-a systematic review and cost analysis. QJM. Oxford Univ Press, 2016;hcw142.
- Ganganah O, Guo SL, Chiniah M, et al. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: A systematic review and meta-analysis. Respirology 2016;21:834-41. [Crossref] [PubMed]
- Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic Lung Cryobiopsy Increases Diagnostic Confidence in the Multidisciplinary Diagnosis of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;193:745-52. [Crossref] [PubMed]
- Yarmus LB, Semaan RW, Arias SA, et al. A randomized controlled trial of a novel sheath cryoprobe for bronchoscopic lung biopsy in a porcine model. Chest 2016;150:329-36. [Crossref] [PubMed]
- Franke KJ, Linzenbold W, Nuessle D, et al. A new tool for transbronchial cryobiopsies in the lung: an experimental feasibility ex vivo study. Respiration 2016;91:228-34. [Crossref] [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [Crossref] [PubMed]
- Tremblay A, Stather DR, MacEachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [Crossref] [PubMed]
- Wong M, Yasufuku K, Nakajima T, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. European Respiratory Journal. Eur Respir J 2007;29:1182-6. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Kurosu K, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis-comparisons with other bronchoscopic diagnostic modalities. Respir Med 2009;103:1796-800. [Crossref] [PubMed]
- Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 2014;146:547-56. [Crossref] [PubMed]
- Kumar S, Chandra S. A. “ROSE” in Every “EBUS” Keeps Transbronchial Lung Biopsy Away. Chest 2014;146:e97. [PubMed]
- Dhooria S, Agarwal R, Aggarwal AN, et al. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: a study of 165 patients. J Thorac Cardiovasc Surg 2014;148:662-7. [Crossref] [PubMed]
- Berim IG, Saeed AI, Awab A, et al. Radial Probe Ultrasound-Guided Cryobiopsy. J Bronchology Interv Pulmonol 2017;24:170-3. [Crossref] [PubMed]


