Medical thoracoscopy and its evolving role in the diagnosis and treatment of pleural disease
Introduction
An estimated 1.5 million patients experience pleural effusions in the United States annually (1). Exudative effusions are very frequently related to underlying malignancy or infections such as tuberculosis, both of which require a timely diagnosis to institute therapy. Thoracentesis is often able to establish a diagnosis and is an appropriate first step, but how to approach undiagnosed exudative pleural effusions remains a source of debate. Due to a number of historical and pragmatic factors, there is considerable diversity in the current approach to this challenge. There is an increasing need to establish an evidence-based algorithm for diagnosing and treating pleural diseases; particularly, better defining the roles of closed pleural biopsy (CPB), medical thoracoscopy (MT), and surgical approaches. Ultimately, it is to the benefit of our patients to consider these options in a multidisciplinary context, integrating the skills and expertise of medical and surgical thoracic specialists to provide a comprehensive approach to pleural disease. Herein, we will consider the non-surgical approaches to diagnosing pleural disease and characterize the current state of evidence supporting their application.
Common terms and equipment
Leon Abrams first reported his experience with his percutaneous “closed” pleural biopsy needle in 1958, and the technique for performing the procedure has changed little in 60 years (2). Conventional CPB has traditionally been performed with either the Abrams or Cope biopsy needles, both of which consist of a hollow trochar with a hooked end through which multiple samples of parietal pleura can be obtained, either with blind technique or under imaging guidance. Cutting needles are stylet-loaded biopsy needles, favored for use with computed tomographic (CT) guidance, and come in a variety of configurations. All of these devices allow for repeated percutaneous sampling of the parietal pleura, but do not offer direct visualization to direct targeted biopsies or the option to perform therapeutic interventions.
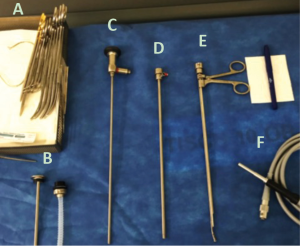
MT also termed “local anesthetic thoracoscopy” and “pleuroscopy,” is a minimally invasive single-port endoscopic technique using rigid and semi-rigid thoracoscopes that offers direct visualization of pleural surfaces, as well as channels to perform diagnostic and therapeutic procedures. Patients typically receive moderate sedation and breathe spontaneously throughout the procedure, without positive pressure ventilation. Whereas the most commonly employed semi-rigid thoracoscopes have a 2.8 mm working channel through which standard flexible biopsy forceps are used, rigid thoracoscopic biopsies are typically obtained with 5 mm rigid biopsy forceps operated through a trochar with an inner diameter of 6–7 mm (3). Furthermore, while semi-rigid thoracoscopes allow for retroflexion and biopsy near the entry site, they offer limited mechanical leverage for sampling tougher, fibrous pleural deposits (3). There are key advantages to each approach, and in the evaluation of pleural disease it is worthwhile to understand factors related to patient selection as well as procedural limitations in deciding on an individualized plan of care.
CPB: indications and moving targets
Pleural fluid cytology plays an important role in the diagnosis of malignancy with a sensitivity of 65% on first sampling and a modest increment with serial sampling, which plateaus around 90% after 3 thoracenteses (4). Serial sampling to diagnose malignancy may delay establishing a diagnosis. Furthermore, the amount of tissue obtained, even from a cell block, may be inadequate or unsuitable for some advanced molecular testing. The sensitivity of pleural cytology alone for diagnosing malignant mesothelioma was estimated at 33% in one series, with a 20% sensitivity for the sarcomatoid subtype (5). When large pleural-based nodules are visible without pleural effusions, imaging-guided CPB is not an unreasonable approach. Without a visible target on CT or ultrasound, the yield of CPB is considerably lower (6,7). Conventional Abrams needle CPB without imaging guidance has a substantially lower sensitivity compared to CT-guided cutting needle biopsy (47% vs. 87%, P=0.02), and when malignancy is strongly suspected in a patient with an exudative effusion, imaging-guided biopsy is preferred if a target exists and thoracoscopy is not an option (8). There appears to be a comparable yield between biopsies performed under live ultrasound-guidance and CT-guidance, and patient-specific factors and operator experience should be considered in deciding upon an approach (9).
Establishing a diagnosis of tuberculous pleurisy remains an important and cost-effective indication for CPB worldwide. Pleuritis from drug-sensitive tuberculosis is typically very responsive to first-line treatment, and interventions (including repeated pleural drainage or pleurodesis) are rarely needed in patients receiving appropriate medical therapy (10). Moreover, tuberculous pleurisy is a disseminated process that lends itself to successful blind biopsy. Diacon et al. found that analyzing pleural fluid adenosine deaminase, lymphocyte/neutrophil ratio, and histologic findings from blind CPB yielded 93% sensitivity for diagnosing pleural tuberculosis in a high-incidence region of South Africa (11). It is clear that the main utility of blind CPB in the modern era is as a cost-effective tool for diagnosing tuberculous pleurisy, particularly in populations with a high pre-test probability.
The role of CPB compared to MT
There is tremendous heterogeneity in approaches to the diagnosis of pleural disease worldwide, largely due to significant differences in training, technical resources, and a lack of high-quality evidence indicating clear superiority of any approach. Attempts to compare CPB to MT have been complicated by differences in the provider’s approach to CPB. Historically conducted as a blind procedure without real-time imaging guidance, CPB has evolved and is increasingly performed under ultrasound guidance by chest physicians or under CT-guidance by interventional radiologists. As a result, comparative studies of MT versus CPB demonstrate variability in the CPB arms owing to these differences (Figure 1). The number of CPBs obtained in trials is also relevant, particularly in diagnosing carcinomas, with an improved diagnostic sensitivity of 89% after four biopsies, as compared to 54% with one biopsy (16).
The largest comparative study of these modalities was a retrospective analysis by Maturu et al. in patients with undiagnosed exudative pleural effusions (12). Over the course of 10 years, CPBs were performed without imaging guidance using either the Abrams or cutting needle techniques and MT was performed with either a rigid or semi-rigid thoracoscope, with or without ultrasound guidance for site selection. The diagnostic yield of MT was higher than CPB (93.2% vs. 84.5%, P=0.02) with a reported yield of 98.7% when ultrasound visualization was used to guide trochar placement in MT (12). They reported a morbidity of 5.6% (including empyema and re-expansion pulmonary edema) with MT compared to 8.3% with CPB (hemothorax, chest wall hematoma), and one death due to sepsis attributed to MT (mortality of 0.37%), with none in the CPB group. These findings were corroborated in a randomized, controlled trial by Haridas et al. comparing blind Abrams needle CPB with MT to establish a diagnosis in 58 patients with an exudative pleural effusion (13). They reported a significantly better diagnostic yield with MT compared to CPB (86.2% vs. 62.1% P=0.036), with a higher rate of reported complications in the CPB arm (17.2% vs. 10.3% in the MT arm), mainly hydropneumothorax. Table 1 summarizes the relative complication rates of these two approaches.
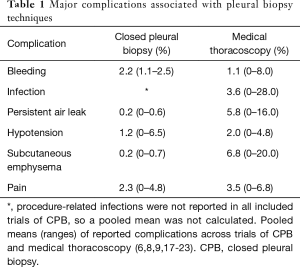
Full table
The utility of MT over imaging-guided CPB was similarly investigated by Metintas et al. in a randomized controlled trial (RCT) of 124 patients with cytology-negative exudative pleural effusions randomized to undergo either CT-guided Abrams needle biopsies or MT (6). The overall sensitivity of MT was 94.1% compared to 87.5% in CT-guided CPB. This trial discriminated features of pleural anatomy on pre-procedure CT and correlated this with sensitivity, observing that in the presence of smooth pleural thickening, the sensitivity of CPB was 73% compared to 100% with MT, particularly with pleura less than 1 cm in thickness. This study suggests that there may be a significant role for MT in identifying flat mucosal abnormalities not evident via non-invasive imaging techniques, and highlights the need for judicious patient selection, given the superior yield of MT in patients with particular pleural characteristics. Furthermore, these studies do not address the therapeutic role of MT during which talc application (poudrage) and simultaneous placement of an ITPC can be accomplished, which cannot be performed during an image-guided CPB approach. As formal training in MT is expanded through dedicated interventional pulmonology fellowship programs, and as techniques are refined and standardized, we anticipate that MT will play a much larger role in the diagnosis and treatment of pleural diseases (24).
MT: techniques, considerations and innovations
Techniques: rigid vs semi-rigid approach
Historically, thoracoscopy has been undertaken with rigid thoracoscopes, as first performed by Hans Christian Jacobaeus in 1910 for the evaluation of tuberculous empyemas (25). While there have been significant advances in the technology employed, the basic principles remain similar to how he described them over a century ago. Despite this, the application of this procedure is remarkably varied between institutions and globally. A current area of deliberation concerns the application of semi-rigid thoracoscopes, which combine the flexibility of bronchoscopes with the stability of rigid thoracoscopes (Figure 2). Proposed advantages of the semi-rigid thoracoscope include ease of maneuverability in the pleural space, a smaller incision site, and possibly lower anesthetic requirements. Additionally, many chest physicians are more comfortable learning to use the semi-rigid thoracoscope because it resembles the flexible bronchoscope in design and operation. Ernst et al. first reported their experience with semi-rigid thoracoscopy in 2002, and since then there have been multiple trials in a variety of settings noting its diagnostic and therapeutic efficacy (26-31). The diagnostic yield of semi-rigid thoracoscopy was well-characterized by Agarwal et al. in their meta-analysis of 17 individual trials involving this approach in 755 patients (32). They calculated an aggregate sensitivity of 91%, specificity of 100% and a rate of major complications of 1.5%, with relatively little evidence of publication bias. Given these remarkable findings, there has been interest in establishing whether there are factors favoring semi-rigid over traditional rigid thoracoscopy.
Small-scale trials of both approaches suggest they have a comparable diagnostic yield, despite the generally larger biopsy specimens obtained via rigid thoracoscopy (32,33). This finding was challenged in the only RCT comparing the two techniques by Dhooria et al., who found that while the diagnostic yield of biopsies obtained was similar (100% with rigid vs. 94.3% with semi-rigid), when they conducted intention-to-treat analysis including cases where a successful biopsy was not obtained, rigid thoracoscopy had a significantly higher yield compared to semi-rigid (100% vs. 73.3%, P=0.02) (17). Patients in this trial randomized to rigid thoracoscopy required slightly more sedation (an additional 1 mg midazolam) to tolerate the procedure and had larger scars (by 5 mm) compared to those undergoing semi-rigid, but these were small differences in absolute terms. The mean size of biopsies obtained by rigid technique was significantly larger (13.9±4.4 vs. 4.4±1.4 mm, P=0.001) with a comparable procedural time. In an era where obtaining adequate tissue is paramount for molecular testing and clinical trials, this difference may be even more important. Operators were also asked to rate their subjective experience of performing each procedure, and they noted that while they felt image quality was better with semi-rigid thoracoscopy, it was significantly easier to take a biopsy with the rigid thoracoscope. Overall, this RCT suggests that when biopsies are obtained, there is little difference in the procedural approach selected, but rigid thoracoscopy remains superior in the setting of difficult-to-biopsy lesions (17).
While rigid and semi-rigid thoracoscopes are well-studied and widely-accepted methods of conducting MT, case reports have been published describing innovations in MT methodology. One such report by Harris et al. described use of a video bronchoscope through a peel-away sheath to obtain parietal pleural biopsies. Whether these newer method confer a therapeutic, diagnostic, cost-saving, or safety advantage over rigid or semi-rigid MT remains to be determined in larger-scale studies (34).
Procedural considerations
Patient factors and anesthetic selection
Patients undergoing MT commonly have at least moderate pleural effusions, and co-morbid pulmonary and extra-pulmonary conditions must be taken into consideration when selecting patients to undergo MT under moderate sedation. Generally, patients must be able to tolerate lying for at least 30 minutes in the lateral decubitus position, with the side of the pleural cavity of interest oriented superiorly. The British Thoracic Society has compiled recommendations for pre-procedural risk assessment prior to MT, and these along with other considerations from our experience and the published literature are summarized in Table 2 (18,19,35-37). Evidence from a large series suggests that common “high risk” clinical features identified prior to MT do not seem to be associated with a higher rate of adverse events (19). Metintas et al. reviewed complications in 355 patients following MT based on the presence of pre-defined “high risk” features including advanced age, hypoxemia, renal failure, cardiomyopathy and a recent venous thromboembolic event (19). In their analysis, the only difference in complications based on “high risk” status was in post-procedural pain (observed in 12.3% of the “high risk” arm vs. 4.4% in the “standard risk”, P=0.007), with no difference in other common complications. Particular complications associated with MT include bleeding, re-expansion pulmonary edema (reported in 2% of patients), malignant seeding of the entry tract (particularly in malignant mesothelioma), empyema, fibrinous pleuritis, and intercostal neuralgia (35,38-40).
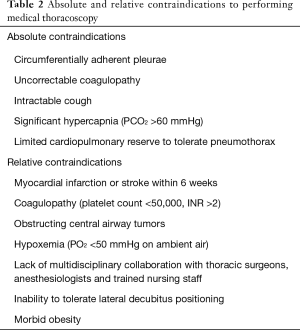
Full table
Underlying hypoventilation may be exacerbated in the 48 h following MT, warranting close monitoring in patients with even mild baseline hypercapnia. Arterial carbon dioxide tension (PCO2) has been reported to increase 10–13 mmHg from baseline 24 h after MT, corresponding with short-term decreases in maximal inspiratory pressure and spirometric measurements (41,42). This is likely multifactorial, owing to the effects of sedatives and analgesics, splinting from post-procedural pain, as well as the potential effects of interventions (e.g., talc poudrage) on the dynamics of the respiratory muscles and the chest wall. It is reasonable to consider these factors when individualizing the approach to procedural sedation. A variety of anesthetic strategies have been employed to conduct MT. These include local analgesics alone, or in combination with intravenous benzodiazepines and opioids, propofol, or regional anesthesia via thoracic paravertebral nerve block, with no consensus on superiority of any approach, though more hypotension and hypoxemia were seen with the use of propofol (43-45). It is clear, however, that MT can be well-tolerated and performed safely with effective local analgesia and minimal intravenous sedation in the majority of patients.
Pleural access
Establishing safe pleural access with a trochar is the first challenge of thoracoscopy, and can be complicated by inadvertent malpositioning, lung puncture, or placement in an area of dense loculation inaccessible to the rigid thoracoscope. One approach to avoid these complications has been the pre-procedural induction of a pneumothorax, with a safe site of entry identified on a decubitus chest radiograph or fluoroscopy, although this technique requires additional procedural time and is not helpful when there are significant fibrous adhesions or a large amount of pleural fluid (46). The use of thoracic ultrasound to identify an optimal access site offers many advantages, including the ability to avoid adhesions, to target sites with best access to pleural-based nodules, to identify the exact depth of lung parenchyma (obviating the need to induce a pneumothorax) and, with color Doppler, to avoid the intercostal vasculature (46-50). Thoracic ultrasound has been successfully used to guide pleural access even in the absence of pleural effusion, targeting sites with “lung sliding” as a sign of free movement of the parietal and visceral pleurae (48). Thoracic ultrasound is significantly better than CT at identifying fibrous septations, and is an important tool in improving the efficacy of thoracoscopy (49). Training in thoracic ultrasound has become a core competency in most interventional pulmonology fellowship programs, and should be considered as standard for guidance of all pleural procedures (51).
Diagnostic applications
With the widening scope of targeted cancer therapies including the checkpoint inhibitor immunotherapies and clinical trials available at many academic medical centers, there is an increasing demand for high quality tumor specimens which MT is well-suited to provide. MT allows for gross visual inspection of both visceral and parietal pleural surfaces (Figure 3), as well as biopsies obtained under direct visualization, accounting for its extremely high diagnostic yield comparable to video assisted thoracoscopic surgery (VATS) (52). Visual inspection alone is often used to determine whether to perform simultaneous therapeutic pleurodesis during MT, but a weak correlation exists between gross and histopathologic findings (53). The addition of autofluorescence during MT to better identify biopsy targets has been explored. In a recent study of 491 parietal pleural biopsy specimens, Wang et al. characterized 37 specimens as “discrepant”, i.e., autofluorescence-positive, but without corresponding changes noted on white light thoracoscopy. Of these 37 lesions, 23 were malignant and 14 demonstrated non-specific inflammation. The sensitivity of autofluorescence (100%) was superior in identifying pleural lesions than that of white light thoracoscopy (92.8%) (54). Narrow band imaging (NBI) is a visual processing technique which has also been explored for application during MT, wherein light captured by the endoscope is filtered for wavelengths which correspond with oxyhemoglobin absorption (55). The result is highlighting of vascular structures to aid in targeting of biopsies. When applied using video pleuroscopy, NBI has shown superiority to conventional white light imaging at identifying irregular vascular patterns, even in flat lesions along the parietal pleura (56). These irregular patterns often correspond with areas of tumor ingrowth, with one comparative trial showing improved accuracy in targeting these sites using NBI versus white light imaging (56). The utility of NBI in improving diagnostic yield outcomes remains unclear overall, though it may be suited to targeting biopsies in patients without obvious pleural tumor implants.

Porfyridis et al. found that visual inspection had a specificity of only 44.7% for correctly predicting a diagnosis (57). The authors explored the use of rapid on-site evaluation (ROSE) during MT, a technique commonly used during bronchoscopic lung cancer staging to determine adequacy of specimens. The area under the curve for ROSE to diagnose malignancy was 0.86 (95% CI, 0.76–0.96; P<0.001). This suggests that, in the setting of recurrent exudative effusions being evaluated by MT, the decision of whether to pursue simultaneous talc pleurodesis may be assisted by ROSE. In practice, many operators have moved away from talc pleurodesis, instead favoring the placement of ITPC at the conclusion of MT. ITPC placement shortens recovery time, allows for same-day discharge, and may avoid the need to establish a diagnosis intraoperatively. Some centers have performed biopsies of visceral pleura and even lung parenchyma during MT, but given the technical complexity and associated risks, these techniques remain limited to highly experienced thoracoscopists and thoracic surgeons (35,58).
Therapeutic applications
The most common therapeutic indication for MT is chemical pleurodesis, typically performed with talc poudrage wherein aerosolized talc powder is applied under direct visualization to the pleural surfaces. Alternative approaches to MT talc poudrage include the gold-standard VATS, placement of ITPC, instillation of talc slurry through a small-bore chest tube ITPC or some combination of these approaches (59). Several trials comparing talc slurry and thoracoscopic poudrage have shown a slight advantage to the thoracoscopic approach, during which adhesiolysis can be performed to improve pleural contact and pleurodesis (60,61). A UK-based group is currently comparing the efficacy of talc slurry and thoracoscopic poudrage in a large-scale multicenter RCT which aims to definitively establish the short- and long-term efficacy, improvement in dyspnea, and cost-effectiveness of these two approaches (62). This trial (the TAPPS trial, ISRCTN47845793) has completed enrollment and results are anticipated to define more clearly the role of thoracoscopic talc poudrage in the management of malignant pleural effusions.
The available evidence suggests that talc poudrage may have a similar rate of pleurodesis compared to ITPC in the setting of malignant effusions, with comparable improvements in dyspnea and health-related quality of life scores (63). The TIME-2 trial also established that the rate of pleurodesis between talc slurry and ITPCs is the same, with more re-interventions required in the talc slurry group (OR: 0.21 for ITPCs; 95% CI, 0.04–0.86; P=0.03) (64). Furthermore, talc pleurodesis, whether by tube thoracostomy or MT, requires 4–7 days of inpatient admission to allow for complete drainage prior to chest tube removal in contrast to ITPC placement after MT, which is commonly done as a same-day procedure without hospitalization (65-69). As reported by other groups, we have had excellent results at our center performing MT with placement of ITPCs in an ambulatory setting, and have been able to simultaneously obtain diagnostic tissue and perform definitive palliation with minimal burden to patients (68). Placement of ITPCs during diagnostic/therapeutic MT may be particularly effective as the biopsy sites in parietal pleura are foci for enhanced pleural inflammation and pleurodesis.
The role of MT in the management of complex parapneumonic effusions has been less rigorously studied than in malignancy. At many centers, experienced operators routinely perform lysis of adhesions and drainage of purulent material from the pleural space (20,21,70). Thoracoscopic visualization can be compromised in empyemas, particularly in the presence of a dense, organized effusion, and there is a risk of bleeding from vessels embedded in organized fibrous septations (71,72). A small retrospective study by Ravaglia et al. suggests that the best efficacy of MT in relieving parapneumonic effusions is seen with free-flowing effusions (100% reported rate of success) and loculated empyema (91.7%), with far less success treating organized effusions (50%) which are better suited for surgical decortication (72). MT offers only a modest diagnostic benefit in the setting of empyema (contributing a microbiologic diagnosis in 47% of patients in one study), as most patients have already completed a significant course of antibiotics prior to intervention (20). Whether there is a benefit of MT in treating empyema over medical management or VATS decortication remains unclear, especially in an era where intrapleural fibrinolytics [tissue plasminogen activator (tPA) and recombinant deoxyribonuclease (DNase)] instilled via tube thoracostomy are routinely used to avoid an invasive procedure.
Procedural competency and training guidelines
There is significant regional variation in practice patterns and training of chest physicians for performing MT and CPB. In the United States, only a small minority of pulmonologists (primarily those undergoing formal training in an interventional pulmonology fellowship) practice MT, and physician competency with CPB has declined with the decreasing incidence of tuberculosis. Centers in Europe and Asia have considerably more experience with MT. Clear training guidelines and competency standards have yet to be developed by any major physician organizations. The British Thoracic Society has recommended levels of safe practice for thoracoscopists, with advanced techniques (including lysis of adhesions, visceral pleural biopsy, lung biopsy, and pneumothorax induction) reserved for “Level II” operators, but no mechanism for establishing competency has been described (35).
As the field of interventional pulmonology has grown into a distinct sub-specialty with formalized fellowship training programs, there is a significant impetus for developing competency-based assessment tools. A survey by Yarmus et al. of interventional pulmonologists and fellows in the United States reported a range of 5–83 medical thoracoscopies performed by fellows during a 1-year training program with a median of 17.5 procedures (73). In total, 50% of responding programs had fewer than the minimum 20 procedures recommended by the American College of Chest Physicians to demonstrate competency. As more centers build their MT programs, a standard evidence-based approach will be necessary. Programs which have initiated MT have demonstrated significant improvements in diagnostic yield over time, and we anticipate that application of this technique will continue to grow in centers with dedicated interventional pulmonology programs (74).
Innovations in MT
Several RCTs currently under way aim to answer key questions about our approach to pleural biopsy. A RCT in India is exploring the use of a “mini-rigid” thoracoscope with a 5.5 mm diameter working channel, comparing diagnostic yield and patient-centered outcomes against the semi-rigid thoracoscope (NCT02851927). Their goal is to determine whether the “mini-rigid” approach has similar efficacy to the traditional rigid thoracoscope, with less procedure-related discomfort. Another group hopes to improve the diagnostic yield of the semi-rigid approach by performing cryobiopsy of parietal pleura with the standard flexible cryoprobe, comparing yield to the standard forceps biopsy (NCT02500277). The semi-rigid thoracoscope offers excellent visualization, but is limited in mechanical leverage, making it an ideal application for the cryobiopsy technique. The REPEAT trial, ongoing in Denmark, hopes to establish the comparability of MT and VATS pleural biopsy, with respect to diagnostic yield and the need for additional interventions in patients with suspected malignancy (NCT02834455). Given the indistinct boundaries between these two procedures, this trial may offer guidance regarding patient selection factors and may better define the complementary roles of medical and surgical thoracic specialists in managing pleural disease. Finally, Majid et al. are exploring the role of MT in the management of complex parapneumonic effusions in a trial comparing the procedure against current standard-of-care medical therapy with combined intrapleural tPA and DNase (NCT02973139). These investigations will help to refine and broaden our application of MT as an important component of our multidisciplinary approach to pleural disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Light RW. Pleural effusions. Med Clin North Am 2011;95:1055-70. [Crossref] [PubMed]
- Abrams LD. A pleural-biopsy punch. Lancet 1958;1:30-1. [Crossref] [PubMed]
- Alraiyes AH, Dhillon SS, Harris K, et al. Medical Thoracoscopy. PLEURA 2016;3:2373997516632752. [Crossref]
- Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol 1994;7:665-8. [PubMed]
- Rakha EA, Patil S, Abdulla K, et al. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagnostic Cytopathology 2010;38:874-9. [Crossref] [PubMed]
- Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest 2010;137:1362-8. [Crossref] [PubMed]
- Botana-Rial M, Leiro-Fernandez V, Represas-Represas C, et al. Thoracic ultrasound-assisted selection for pleural biopsy with Abrams needle. Respir Care 2013;58:1949-54. [Crossref] [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [Crossref] [PubMed]
- Khosla R, McLean AW, Smith JA. Ultrasound-guided versus computed tomography-scan guided biopsy of pleural-based lung lesions. Lung India 2016;33:487-92. [Crossref] [PubMed]
- Zhai K, Lu Y, Shi HZ. Tuberculous pleural effusion. J Thorac Dis 2016;8:E486-94. [Crossref] [PubMed]
- Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589-91. [Crossref] [PubMed]
- Maturu VN, Dhooria S, Bal A, et al. Role of medical thoracoscopy and closed-blind pleural biopsy in undiagnosed exudative pleural effusions: a single-center experience of 348 patients. J Bronchology Interv Pulmonol 2015;22:121-9. [Crossref] [PubMed]
- Haridas N. Medical Thoracoscopy vs Closed Pleural Biopsy in Pleural Effusions: A Randomized Controlled Study. J Clin Diagn Res 2014;8:MC01-4. [PubMed]
- Mishra AK, Verma SK, Kant S, et al. A study to compare the diagnostic efficacy of closed pleural biopsy with that of the thoracoscopic guided pleural biopsy in patients of pleural effusion. South Asian J Cancer 2016;5:27-8. [Crossref] [PubMed]
- Mohamed AS, Abo-Sheisha DM, Shamloula MM. Exudative pleural effusions: Comparative study of image assisted Abram needle pleural biopsy and medical thoracoscopy. Egypt J Chest Dis Tuberc 2014;63:625-8. [Crossref]
- Jiménez D, Perez-Rodriguez E, Diaz G, et al. Determining the optimal number of specimens to obtain with needle biopsy of the pleura. Respir Med 2002;96:14-7. [Crossref] [PubMed]
- Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014;59:756-64. [Crossref] [PubMed]
- Hansen M, Faurschou P, Clementsen P. Medical thoracoscopy, results and complications in 146 patients: a retrospective study. Respir Med 1998;92:228-32. [Crossref] [PubMed]
- Metintas M, Ak G, Yildirim H, et al. The safety of medical thoracoscopy in a group at high risk for complications. J Bronchology Interv Pulmonol 2013;20:224-31. [Crossref] [PubMed]
- Brutsche MH, Tassi GF, Gyorik S, et al. Treatment of sonographically stratified multiloculated thoracic empyema by medical thoracoscopy. Chest 2005;128:3303-9. [Crossref] [PubMed]
- Hardavella G, Papakonstantinou NA, Karampinis I, et al. Hippocrates Quoted "If an Empyema Does Not Rupture, Death Will Occur": Is Medical Thoracoscopy Able to Make It Rupture Safely? J Bronchology Interv Pulmonol 2017;24:15-20. [Crossref] [PubMed]
- Metintas M, Yildirim H, Kaya T, et al. CT Scan-Guided Abrams' Needle Pleural Biopsy versus Ultrasound-Assisted Cutting Needle Pleural Biopsy for Diagnosis in Patients with Pleural Effusion: A Randomized, Controlled Trial. Respiration 2016;91:156-63. [Crossref] [PubMed]
- Niu XK, Bhetuwal A, Yang HF. CT-Guided Core Needle Biopsy of Pleural Lesions: Evaluating Diagnostic Yield and Associated Complications. Korean J Radiol 2015;16:206-12. [Crossref] [PubMed]
- Egressy K, Murgu S. Medical thoracoscopy versus closed pleural biopsy: should this saga continue in the era of competency-oriented training? J Bronchology Interv Pulmonol 2015;22:95-6. [Crossref] [PubMed]
- Marchetti GP, Pinelli V, Tassi GF. 100 years of thoracoscopy: historical notes. Respiration 2011;82:187-92. [Crossref] [PubMed]
- Ernst A, Hersh CP, Herth F, et al. A novel instrument for the evaluation of the pleural space: an experience in 34 patients. Chest 2002;122:1530-4. [Crossref] [PubMed]
- Nattusamy L, Madan K, Mohan A, et al. Utility of semi-rigid thoracoscopy in undiagnosed exudative pleural effusion. Lung India 2015;32:119-26. [Crossref] [PubMed]
- Karasulu AL, Dalar L, Altin S, et al. Semirigid thoracoscopy in the diagnosis of pleural effusion: first four cases in Turkey. Tuberk Toraks 2011;59:188-93. [Crossref] [PubMed]
- Rozman A, Camlek L, Kern I, et al. Semirigid thoracoscopy: an effective method for diagnosing pleural malignancies. Radiology and Oncology 2014;48:67-71. [Crossref] [PubMed]
- Willendrup F, Bodtger U, Colella S, et al. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions in Denmark. J Bronchology Interv Pulmonol 2014;21:215-9. [Crossref] [PubMed]
- Munavvar M, Khan MA, Edwards J, et al. The autoclavable semirigid thoracoscope: the way forward in pleural disease? Eur Respir J 2007;29:571-4. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest 2013;144:1857-67. [Crossref] [PubMed]
- Khan MA, Ambalavanan S, Thomson D, et al. A comparison of the diagnostic yield of rigid and semirigid thoracoscopes. J Bronchology Interv Pulmonol 2012;19:98-101. [Crossref] [PubMed]
- Harris K, Singh Dhillon S, Alraiyes AH. Medical Pleuroscopy Using a Peel-Away Introducer Sheath and a Hybrid Bronchovideoscope. Ann Am Thorac Soc 2016;13:976-8. [Crossref] [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii54. [Crossref] [PubMed]
- Rodríguez-Panadero F. Medical thoracoscopy. Respiration 2008;76:363-72. [Crossref] [PubMed]
- Kern RM, DePew ZS, Maldonado F. Outpatient thoracoscopy: safety and practical considerations. Curr Opin Pulm Med 2015;21:357-62. [Crossref] [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [Crossref] [PubMed]
- Metintas M, Ak G, Cadirci O, et al. Outcome of patients diagnosed with fibrinous pleuritis after medical thoracoscopy. Respir Med 2012;106:1177-83. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Deaths and complications associated with respiratory endoscopy: a survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2012;17:478-85. [Crossref] [PubMed]
- Froudarakis ME, Pataka A, Makris D, et al. Respiratory muscle strength and lung function in patients undergoing medical thoracoscopy. Respiration 2010;80:220-7. [Crossref] [PubMed]
- Chhajed PN, Kaegi B, Rajasekaran R, et al. Detection of hypoventilation during thoracoscopy: combined cutaneous carbon dioxide tension and oximetry monitoring with a new digital sensor. Chest 2005;127:585-8. [Crossref] [PubMed]
- Agnoletti V, Gurioli C, Piraccini E, et al. Efficacy and safety of thoracic paravertebral block for medical thoracoscopy. Br J Anaesth 2011;106:916-7. [Crossref] [PubMed]
- Tschopp JM, Purek L, Frey JG, et al. Titrated sedation with propofol for medical thoracoscopy: a feasibility and safety study. Respiration 2011;82:451-7. [Crossref] [PubMed]
- Grendelmeier P, Tamm M, Jahn K, et al. Propofol versus midazolam in medical thoracoscopy: a randomized, noninferiority trial. Respiration 2014;88:126-36. [Crossref] [PubMed]
- Macha HN, Reichle G, von Zwehl D, et al. The role of ultrasound assisted thoracoscopy in the diagnosis of pleural disease. Clinical experience in 687 cases. Eur J Cardiothorac Surg 1993;7:19-22. [Crossref] [PubMed]
- Hersh CP, Feller-Kopman D, Wahidi M, et al. Ultrasound guidance for medical thoracoscopy: a novel approach. Respiration 2003;70:299-301. [Crossref] [PubMed]
- Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest 2015;147:1008-12. [Crossref] [PubMed]
- Medford ARL, Agrawal S, Bennett JA, et al. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts fibrous septation. Respirology 2010;15:804-8. [Crossref] [PubMed]
- Shoukri A. Ultrasound-assisted medical thoracoscopy. Egyptian Journal of Bronchology 2015;9:92-5.
- Williamson JP, Twaddell SH, Lee YC, et al. Thoracic ultrasound recognition of competence: A position paper of the Thoracic Society of Australia and New Zealand. Respirology 2017;22:405-8. [Crossref] [PubMed]
- Ferreiro L, Suarez-Antelo J, Valdes L. Pleural procedures in the management of malignant effusions. Ann Thorac Med 2017;12:3-10. [Crossref] [PubMed]
- Hallifax RJ, Corcoran JP, Psallidas I, et al. Medical thoracoscopy: Survey of current practice-How successful are medical thoracoscopists at predicting malignancy? Respirology 2016;21:958-60. [Crossref] [PubMed]
- Wang F, Wang Z, Tong Z, et al. A pilot study of autofluorescence in the diagnosis of pleural disease. Chest 2015;147:1395-400. [Crossref] [PubMed]
- Schönfeld N, Schwarz C, Kollmeier J, et al. Narrow band imaging (NBI) during medical thoracoscopy: first impressions. J Occup Med Toxicol 2009;4:24. [Crossref] [PubMed]
- Ishida A, Ishikawa F, Nakamura M, et al. Narrow band imaging applied to pleuroscopy for the assessment of vascular patterns of the pleura. Respiration 2009;78:432-9. [Crossref] [PubMed]
- Porfyridis I, Georgiadis G, Michael M, et al. Rapid on-site evaluation with the Hemacolor rapid staining method of medical thoracoscopy biopsy specimens for the management of pleural disease. Respirology 2016;21:1106-12. [Crossref] [PubMed]
- Tassi GF, Davies RJ, Noppen M. Advanced techniques in medical thoracoscopy. Eur Respir J 2006;28:1051-9. [Crossref] [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [Crossref] [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Clive AO, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2016.CD010529. [PubMed]
- Bhatnagar R, Laskawiec-Szkonter M, Piotrowska HE, et al. Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry (TAPPS trial): protocol of an open-label randomised controlled trial. BMJ Open 2014;4:e007045. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 263-4.
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. Jama 2012;307:2383-9. [Crossref] [PubMed]
- Bhatnagar R, Reid ED, Corcoran JP, et al. Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax 2014;69:959-61. [Crossref] [PubMed]
- Fortin M, Tremblay A. Pleural controversies: indwelling pleural catheter vs. pleurodesis for malignant pleural effusions. J Thorac Dis 2015;7:1052-7. [PubMed]
- Psallidas I, Corcoran JP, Fallon J, et al. Provision of day-case local anesthetic thoracoscopy: A multicenter review of practice. Chest 2017;151:511-2. [Crossref] [PubMed]
- DePew ZS, Wigle D, Mullon JJ, et al. Feasibility and safety of outpatient medical thoracoscopy at a large tertiary medical center: a collaborative medical-surgical initiative. Chest 2014;146:398-405. [Crossref] [PubMed]
- Medford AR, Agrawal S, Free CM, et al. A local anaesthetic video-assisted thoracoscopy service: prospective performance analysis in a UK tertiary respiratory centre. Lung Cancer 2009;66:355-8. [Crossref] [PubMed]
- Xiong Y, Gao X, Zhu H, et al. Role of medical thoracoscopy in the treatment of tuberculous pleural effusion. J Thorac Dis 2016;8:52-60. [PubMed]
- Breen DP, Daneshvar C. Role of interventional pulmonology in the management of complicated parapneumonic pleural effusions and empyema. Respirology 2014;19:970-8. [Crossref] [PubMed]
- Ravaglia C, Gurioli C, Tomassetti S, et al. Is medical thoracoscopy efficient in the management of multiloculated and organized thoracic empyema? Respiration 2012;84:219-24. [Crossref] [PubMed]
- Yarmus L, Feller-Kopman D, Imad M, et al. Procedural volume and structure of interventional pulmonary fellowships: a survey of fellows and fellowship program directors. Chest 2013;144:935-9. [Crossref] [PubMed]
- Valsecchi A, Arondi S, Marchetti G. Medical thoracoscopy: Analysis on diagnostic yield through 30 years of experience. Ann Thorac Med 2016;11:177-82. [Crossref] [PubMed]



