A rare mixed breast cancer of intraductal and solid papillary with tubular carcinoma histotypes in a young woman
Introduction
Tumors with mixed histology account for less than 8% of total breast cancer diagnoses (1,2); even rarer is mixed breast cancer with rare carcinoma histotypes, including papillary (intraductal, intracystic, or solid), mucinous, medullary, and tubular carcinoma cells (3). The low incidence of the malignancies hinders research attempts to understand etiopathology and progression of the disease. We here reported the first case of mixed breast cancer combining histotypes of intraductal and solid papillary as well as tubular carcinoma in a 26-year-old woman.
Case presentation
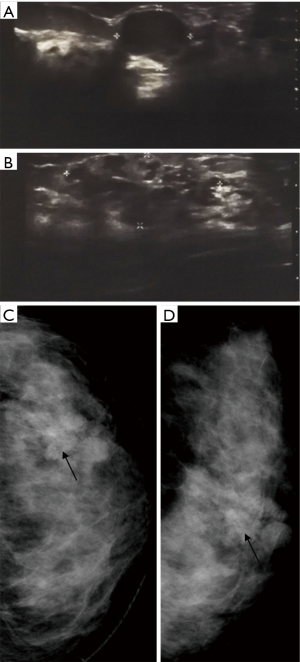
A 26-year-old woman, diagnosed with cancer of the left breast and scheduled for a radical mastectomy at another hospital, visited our clinic for a review. The patient presented with a painless lump first noticed 3 years before as a 2-cm but slowly growing mass, in the lateral hemisphere of her left breast. Having no family history of any cancer, the patient showed no symptoms of inverted nipple, nipple discharge, or skin dimpling, and no abnormalities in biochemical and hematological parameters. Clinical examination revealed a firm, but movable, bilobed mass in the left breast, without axillary lymphadenopathy. Core needle biopsy was not performed due to the patient’s concern over cancer cell spreading. Ultrasound scanning showed a 1.5×1.5×1.2 cm3 hypoechoic area with indistinct borders at the 3 o’clock position in the left breast, 2 cm from the nipple (Figure 1A). Further laterally, 4 cm from the nipple, a bosselated disruption (3.2×2.4×1.5 cm3) was detected with a tubular structure and an intraluminal hypoechoic signal (Figure 1B). Both penetrating and peripheral tumor vascularity was detected. The tumor was classified a Category 4b in the breast imaging report and data system (BI RADS), and mammography revealed disorganized glandular structures with a Category 4 BI-RADS tumor (Figure 1C,D). Examination of the right breast and lymph nodes revealed no cystic or solid nodules or axillary lymphadenopathy.
Wide local excision surgery was performed, and intraoperative observation showed a 3-cm tumor in the left lateral hemisphere connected with a 2-cm medially located mass, forming a bilobed pattern. Examination of the frozen sections showed intraductal papillary tumor elements in both nodules, and evidence of intraductal papillary carcinoma in the medial nodule, whereas the tumor margins were clear. A week later, histopathologic examination of paraffin-embedded sections confirmed intraductal papillary carcinoma (Figure 2A) of the medial nodule and a mixture of solid papillary (Figure 2B) and tubular carcinoma (Figure 2C) in the lateral mass. A sentinel lymph node biopsy of the axillary lymph nodes revealed no metastasis among all four nodes examined.
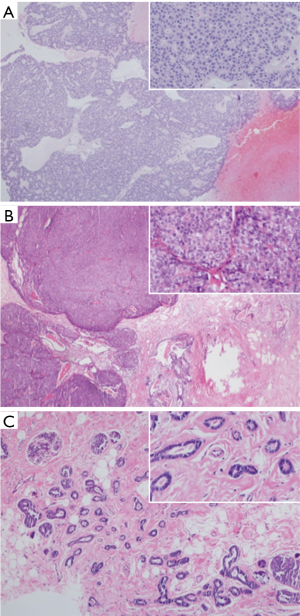
The medial nodule exhibited arrangement of cancer cells in sheets or fused ductal structures along the fibrovascular cores (Figure 2A) while the lateral tumor showed features of solid papillary carcinoma (Figure 2B), with adjacent solid nests of varying sizes separated by fibrous walls of variable thickness. The nested structures were round with distinct borders and filled with morphologically uniform cells grouped into sheets, between which a capillary vasculature was visible. Moreover, tubular carcinoma of the lateral nodule, characterized by the intralobular, randomly organized tubular structures segregated by fibrohyaline stroma, was observed. These tubules contained a single layer of epithelial cells, organized into irregular shapes with open lumens (Figure 2C).
Immunohistochemical analysis suggested that both nodules were negative for myoepithelial cell marker p63. Further, the medial tumor tested negative for estrogen receptors (ER), progesterone receptors (PR), epidermal growth factor receptor (HER) 2, and CD10 (data not shown), whereas the lateral tumor tested negative for ER, PR, HER2, CD10, and cytokeratin (CK) 5/6, but positive for Ki67 (30%) and p53. Following breast conservation surgery, the patient underwent whole-breast irradiation (46 Gy/23 fractions/4.5 weeks) and local intensity-modulated radiation (14 Gy/7 fractions/1.5 weeks) as well as 4 cycles of epirubicin (75 mg/m2) and cyclophosphamide (600 mg/m2), which were undertaken under ovarian suppression using luteinizing hormone-releasing hormone agonists (3.6 mg goserelin/4 weeks) to preserve ovarian function and fertility. The patient tolerated the treatments well, with no febrile neutropenia, gastrointestinal side effects, or significant weight loss, although a 1- to 2-degree decrease of white blood cells was noticed.
At the 1-year follow-up, the surgical wound had healed well and the breast shape was maintained. The patient exhibited no apparent decrease in body weight or signs of a recurrent tumor, and menstrual regularity had been restored.
Discussion
Mixed breast cancer usually comprises common histotypes such as invasive ductal and lobular carcinoma (1,2). With the low incidence of uncommon histotypes, such as solid papillary carcinoma and tubular carcinoma, the coexistence of them in a single mass is extremely rare. In the condition of limited cases and experiences, oncological clinicians should always be alert to recognize these uncommon morphological mixtures and apply the precise and individual treatment according to the different histological components mixed. The case presented here is the first with intraductal and solid papillary and tubular carcinoma within a single lesion.
Papillary and tubular carcinomas represent two uncommon subcategories of breast carcinoma (3) usually diagnosed in elderly patients (4). Intraductal papillary carcinomas mostly grow within dilated ducts, with occasional involvement of adjacent ducts to form a small non-solid mass (5). Immunohistochemically, negative staining for p63 and high-molecular weight CK and positive staining for neuroendocrine markers have been recommended to evaluate malignant potential in intraductal papillary carcinoma (6).
Solid papillary carcinomas manifest a specific type of papillary transformation (5,7). Usually palpable and centrally located with bloody nipple discharge, they appear as round or oval well-circumscribed nodules on mammography and produce hypoechoic signals on ultrasonography (4). Microscopically, the tumor comprises tightly arranged solid nests of invasive carcinoma cells separated by fibrous tissues of irregular thickness. These nests are round, with a distinct border, and filled with low-grade ductal cells arranged in solid sheets segregated by the fibrovascular structure. The observations in the present case conformed to these features. Similar to the intraductal papillary carcinoma, solid papillary carcinomas are usually p63 negative. Nodal metastasis of solid papillary carcinoma is uncommon, consistent with its classification as an in situ carcinoma in the new World Health Organization guideline, when no definite invasion is found (7). However, this tumor may actually represent solid nests of invasive carcinoma instead of a form of in situ carcinoma (8) and, therefore, require lymph node biopsy.
In our patient, tubular carcinoma co-occurred with solid papillary carcinoma in one of the nodules. Tubular carcinoma is a well-differentiated invasive carcinoma with epithelial cells arranged in a typical single-cell columnar architecture, surrounded by abundant fibrohyaline stroma (9). These tumors are mostly positive for hormone receptors but negative for HER2 (10) and p63, suggesting the absence of a myoepithelial envelope. In addition, the absence of S100 staining differentiates tubular carcinoma from microglandular adenosis (11). In the present case, a chronic mobile lump—a feature of benign tumors (12), was found, a risk of misdiagnosis of this histotype. Large tumors with bilobed or phyllode shapes or polyclonal histology can be malignant. A definitive diagnosis requires histopathologic and immunohistochemical analysis of postoperative samples.
Complete excision of the tumor or mastectomy, together with adjuvant therapies, is the standard treatment for papillary and tubular carcinomas (4,13,14), therefore, a wide local excision was carried out in this study. A subsequent sentinel lymph node biopsy was also performed to check for metastasis. Whole-breast irradiation and local intensity-modulated radiation were given as routine after breast-conserving surgery. Adjuvant chemotherapy was undertaken due to negativity for ER, PR, and HER2, and positive staining for Ki-67 in this patient. In cases with ER- and PR-positive tumors without axillary lymphatic metastases, adjuvant endocrine therapies as monotherapy could be an option for systemic treatment (15).
To date, there are no data reported publicly about metastatic pattern and long-term clinical outcome of mixed breast cancer. Generally, we think the prognosis should be related to the worst one in the mix which is determined by tumor size and tumor histotype. In our “3-in-1” case, the future risk is determined by the solid papillary carcinoma and tubular carcinoma whose possibilities of metastasis are both low.
In summary, this report presents a case of breast cancer with mixed papillary (intraductal and solid) and tubular carcinoma in a 26-year-old woman. The patient was diagnosed by clinical examination, imaging, and histopathologic analysis, and treated by breast conserving surgery and sentinel lymph node biopsy followed by adjuvant therapies and radiotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bharat A, Gao F, Margenthaler JA. Tumor characteristics and patient outcomes are similar between invasive lobular and mixed invasive ductal/lobular breast cancers but differ from pure invasive ductal breast cancers. Am J Surg 2009;198:516-9. [Crossref] [PubMed]
- Li CI, Anderson BO, Daling JR, et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 2003;289:1421-4. [Crossref] [PubMed]
- Linda A, Zuiani C, Girometti R, et al. Unusual malignant tumors of the breast: MRI features and pathologic correlation. Eur J Radiol 2010;75:178-84. [Crossref] [PubMed]
- Saremian J, Rosa M. Solid papillary carcinoma of the breast: a pathologically and clinically distinct breast tumor. Arch Pathol Lab Med 2012;136:1308-11. [Crossref] [PubMed]
- Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. International Agency for Research on Cancer, 2003.
- Moritani S, Ichihara S, Kushima R, et al. Myoepithelial cells in solid variant of intraductal papillary carcinoma of the breast: a potential diagnostic pitfall and a proposal of an immunohistochemical panel in the differential diagnosis with intraductal papilloma with usual ductal hyperplasia. Virchows Arch 2007;450:539-47. [Crossref] [PubMed]
- Lakhani SR. WHO classification of tumours of the breast. International Agency for Research on Cancer, 2012.
- Rakha EA, Lee AH, Evans AJ, et al. Tubular carcinoma of the breast: further evidence to support its excellent prognosis. J Clin Oncol 2010;28:99-104. [Crossref] [PubMed]
- Sullivan T, Raad RA, Goldberg S, et al. Tubular carcinoma of the breast: a retrospective analysis and review of the literature. Breast Cancer Res Treat 2005;93:199-205. [Crossref] [PubMed]
- Dieci MV, Orvieto E, Dominici M, et al. Rare breast cancer subtypes: histological, molecular, and clinical peculiarities. Oncologist 2014;19:805-13. [Crossref] [PubMed]
- Salarieh A, Sneige N. Breast carcinoma arising in microglandular adenosis: a review of the literature. Arch Pathol Lab Med 2007;131:1397-9. [PubMed]
- Wilkinson S, Forrest AP. Fibro-adenoma of the breast. Br J Surg 1985;72:838-40. [Crossref] [PubMed]
- Min Y, Bae SY, Lee HC, et al. Tubular carcinoma of the breast: clinicopathologic features and survival outcome compared with ductal carcinoma in situ. J Breast Cancer 2013;16:404-9. [Crossref] [PubMed]
- Javid SH, Smith BL, Mayer E, et al. Tubular carcinoma of the breast: results of a large contemporary series. Am J Surg 2009;197:674-7. [Crossref] [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]


