The pivotal role of pathology in the management of lung cancer
Introduction
Lung cancer remains one of the leading causes of cancer mortality worldwide (1). In Australia in 2009, cancer accounted for 29.8% of all deaths, second only to diseases of the circulatory system, with malignancies of the trachea, bronchus and lungs being the leading cause of cancer related deaths in males (20.1%) and surpassing breast cancer in females (16.5%) (2). Currently, surgical resection with curative intent is the primary treatment for lung cancer, however the vast majority of patients present at an advanced disease stage where medical therapy is the only therapeutic option available. The prognosis for patients diagnosed with lung cancer is poor, with overall five year survival remaining below 15% (3-7). This is partly attributable to relatively ineffective methods for early detection and lack of curative treatment for advanced disease.
However, the last decade has seen rapid development of advanced molecular biology techniques for the study of lung and other cancers, and our understanding and appreciation of the complexity of tumour biology has increased exponentially. It is well established that lung cancer is the result of multiple complex combinations of morphological, molecular and genetic alterations, ultimately leading to a malignant mass of cells bearing the phenotypic ‘hallmarks’ of cancer (8). Accumulation of multiple molecular transformations ultimately results in an imbalance between tumour suppressor genes (TSG) and tumour promoting oncogenes, providing a cell with the potential to become malignant (9). In particular, the acquisition of somatic mutations in critical oncogenes has emerged as potential ‘driver’ events in lung carcinogenesis, and has led to the concept of ‘oncogene addiction’ (10,11). Identification and characterization of such ‘driver’ events has contributed to the development of targeted therapies specific to particular subtypes of lung cancer. Improved patient outcomes as a result of these advances requires multidisciplinary planning of diagnosis and treatment and has significantly expanded the role of the surgical pathologist who must not only confirm the diagnosis of malignancy, but also accurately subtype the tumour based on its histology and molecular profile. As the majority of lung cancer is diagnosed on small biopsies or cytology specimens, often obtained by increasingly sophisticated diagnostic procedures, the pathologist must obtain maximal diagnostic yield from these small and valuable tissue samples.
This review will present an overview of recent developments in the histological classification of lung cancer, and address the challenges facing the surgical pathologist in the era of personalized treatment for lung cancer.
Classification of lung cancer
The World Health Organisation (WHO) classification applies to surgically resected malignant tumours of the lung and pleura (12). Primary carcinomas of the lung are traditionally classified as either small cell lung cancer (SCLC) or non small cell lung cancer (NSCLC). NSCLC constitutes approximately 80% of all primary lung cancers with adenocarcinoma, squamous cell carcinoma (SCC) and large cell carcinoma constituting the major histological types (13,14). The recent revision of the lung adenocarcinoma classification by IASLC/ATS/ERS reflects not only histology, but also pathogenesis (preneoplasia) and clinical behaviour, and refines the classification for application to lung cancer diagnosis in small biopsies and cytology specimens.
Preneoplasia
The development and progression of preneoplastic lesions of the lung continue to generate research interest not only to develop methods for early detection but also to increase our understanding of tumour biology. It is well known that lung cancer is the result of multiple complex combinations of morphological, molecular and genetic alterations. Co-localisation of genetic changes within morphologically abnormal epithelial regions has been convincingly demonstrated and there is evidence that a series of key genetic alterations results in progression through increasingly abnormal morphology, eventually leading to invasive lung carcinoma (15-20).
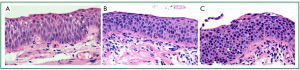
Pulmonary SCC, which typically arises from the bronchial epithelium of the larger, more central airways, progresses through a series of preinvasive neoplastic lesions, from squamous metaplasia, to squamous dysplasia (mild, moderate and severe) and finally carcinoma in situ (CIS) (12,21) (Figure 1A-C). Multiple molecular alterations contribute to this multistage progression, including loss of heterozygosity at 3p21 (an early event), 9p21, 8p22-24, 5q22 and 17p, deregulation of telomerase activity, p53 mutation and deregulation of cell proliferation (cyclin D1 and E) and apoptosis (Bcl-2) (reviewed by Wistuba and Gazdar 2006; Lantuejoul et al. 2009) (22,23).
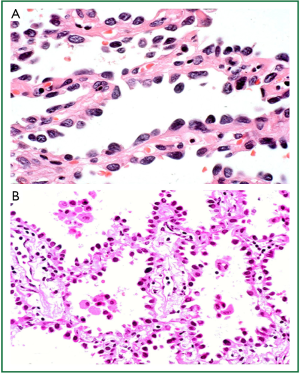
Conversely, lung adenocarcinomas are predominantly more peripheral tumours, thought to arise from the alveolar or bronchiolar epithelium (pneumocytes or Clara cells) (22). The new IASLC/ATS/ERS classification recognises preinvasive adenocarcinoma lesions to include atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS) (21) (Figure 2). The molecular alterations in these lesions are not as well characterised as their counterparts in squamous carcinogenesis, but it is thought that non-smokers progress through alterations in epidermal growth factor receptor (EGFR) signalling whilst smokers progress through alterations in v-Ki-ras2 Kirsten Rat Sarcoma viral oncogene (KRAS) signalling pathways (reviewed by Wistuba and Gazdar 2006) (22). Reflecting the fact that AIS is a preinvasive lesion, complete resection results in 100% 5-year survival (24-30).
For other tumours of the lung, the preneoplastic processes leading to the development of an invasive tumour are not well defined. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) is thought to be a precursor lesion for carcinoid tumours (12,21,22,31). This is a rare lesion of the distal airways characterised histologically by generalised proliferation of neuroendocrine cells as single cells, small nodules or linear proliferations, either confined to the luminal epithelium or forming extraluminal tumorlets and may be associated with fibrosis (12,22). A definite preneoplastic lesion has not been identified for other neuroendocrine tumours of the lung. However, it has been shown that bronchial epithelium adjacent to SCLC demonstrates genetic alterations, even if morphologically normal (22,32). Therefore it has been proposed that SCLC bypasses the traditional multistage preneoplastic sequence and arises directly from epithelium that demonstrates none or only minimal atypia (22,32).
Why subtype non-small cell lung cancer?
Specific subtypes of NSCLC display varying responses to different chemotherapeutic agents. Key oncogenic ‘driver’ events in lung adenocarcinomas include mutually exclusive activating mutations of KRAS and EGFR (33). The discovery of activating mutations in EGFR (exons 18-21) led to subsequent development of EGFR tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib and erlotinib, revolutionizing the management of patients whose tumours harbor these mutations (33-36). Of note, KRAS mutations occur almost exclusively in smokers with adenocarcinoma histology, whilst EGFR mutations are associated with never smoking, adenocarcinoma histology, female gender, and Asian ethnicity, with smoking status possibly the strongest clinical predictor of response to EGFR-TKIs (37-40). This is largely a reflection of the major clinicopathological and molecular differences in lung tumours arising in never smokers compared to smokers, supporting the current theory that they are unique diseases (reviewed by Sun et al., 2007) (40).
A proportion of lung adenocarcinomas show translocations involving the ALK gene (encoding a tyrosine kinase) and a number of partners (most often EML4), resulting in overexpression of the oncogenic ALK protein (41-44). EGFR-TKIs (gefitinib and erlotinib) and ALK-TKIs (crizotinib) are now recommended as first line therapy for patients with EGFR mutations and ALK translocations respectively (45). The IASLC in conjunction with the College of American Pathologists (CAP) and Association for Molecular Pathology (AMP) have published guidelines for EGFR mutation and ALK translocation testing, recommending that all lung adenocarcinomas, regardless of clinical characteristics, undergo validation molecular testing for EGFR mutation and ALK translocation (46). Other potential driver ‘events’ in NSCLC include mutations in KRAS, BRAF, HER2 and FGFR1 (33,47-52). Targeted inhibitors of many of these genes are in various stages of clinical development and may become available in the future as targeted therapies for which additional molecular testing will be required in the diagnostic workup of NSCLC patients.
Not only can molecular biomarkers be used to identify patients that are most likely to benefit from specific targeted molecular therapy, but they can also assist in predicting response to therapeutic agents. Initial studies have shown that levels of thymidylate synthase (TS), the principal enzymatic target of pemetrexed, are high in SCLC and SCC but low in adenocarcinoma (53-55). The observed reduced efficacy of pemetrexed in SCLC and lung SCC compared to adenocarcinoma in clinical trials is likely to be the result of the higher levels of TS (56) and thus it has been suggested that levels of TS expression may be useful as a predictor of response to pemetrexed (55). Similarly, preliminary studies have indicated that ERCC1 protein expression may be a useful biomarker of clinical response to platinum-based chemotherapy (57,58). Despite their potential to assist in personalised targeted therapy for lung cancer patients, the majority of these biomarkers are still at an early stage of development.
Tumour histology of itself may predict response to therapy. Treatment with the vascular endothelial growth factor (VEGF) inhibitor, bevacizumab, has been reported to precipitate life-threatening pulmonary haemorrhage in patients with SCC (59). This has resulted in exclusion of patients whose tumours show squamous differentiation from being treated with this drug or with pemetrexed.
Squamous cell carcinoma
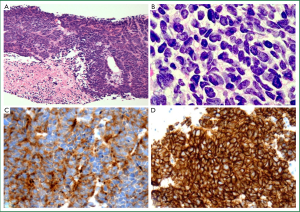
In recent decades the proportion of NSCLC represented by SCC has declined, with current reports estimating that it accounts for approximately 33% worldwide (13). This change is thought to be partly attributable to changes in smoking behaviours. Classically, SCC is a central lung tumour (Figure 3A), however, a significant proportion is identified in the periphery (30). The characteristic morphological features of squamous differentiation include intercellular bridges, individual cell keratinisation and squamous pearl formation (14) (Figure 3B-D). The current WHO classification includes papillary, clear cell, small cell and basaloid subtypes of SCC (12). Apart from basaloid SCC, these subtypes are descriptive only with no proven clinical or prognostic utility.
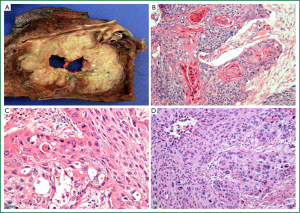
Several publications have suggested alternate approaches to the subclassification of SCC. Maeshima et al. [2006] found that tumours showing single cell infiltration had worse prognosis than those with large (>6 cells) or small (2-5 cells) invasive tumour cell nests (60). A recent review by Travis [2011] proposed abolition of the small cell descriptor because of confusion with true SCLC, and noted overlap of the small cell variant with the basaloid variant (61). Future subclassification of SCC will require meaningful clinicopathological collaboration to establish relevant predictors of treatment response and prognosis.
Adenocarcinoma
Adenocarcinoma now represents the dominant histological subtype of all lung cancers, and in particular is the most common lung tumour in non-smokers, females and Asian patients (62,63). They predominantly arise peripherally (Figure 4A) and are histologically characterised by the presence of glandular differentiation and/or mucin production (12,21). In 2011, the collaborative efforts of the IASLC/ATS/ERS proposed a new subclassification for surgically resected lung adenocarcinoma (21). Of note, the confusing term, bronchioloalveolar carcinoma (BAC), is discarded. The classification introduces the terms AIS (previously BAC) and minimally invasive adenocarcinoma (MIA), both having 5-year survival rates approaching 100% if completely resected (24-30). MIA is defined as a lepidic predominant tumour of less than 3 cm diameter with 5 mm or less of an invasive component (21). Histologically these lesions can be non-mucinous or rarely mucinous and have characteristic radiological appearances (21).
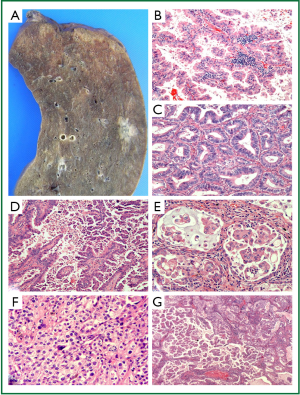
For surgically resected invasive adenocarcinoma, the new IASLC/ATS/ERS classification introduces some important changes reflecting the heterogeneous nature of these tumours. Because the great majority displays mixed histological patterns, it is recommended that the predominant pattern (lepidic, acinar, papillary, micropapillary or solid—see Figure 4B-F) be recorded, with all other subpatterns listed in the pathology report with estimated percentages in 5% increments (21) (Figure 4G). The micropapillary pattern is included for the first time due to multiple studies reporting an association with poor prognosis in early stage lung adenocarcinoma (21,61,64-66). Clear cell and signet ring are no longer included as histological subtypes of adenocarcinoma, but instead are considered as cytological variants seen in many subtypes of lung cancer; their presence may still be reported (21,61). This algorithm for reporting of lung adenocarcinoma allows for inclusion of small components that may hold prognostic implications (e.g., micropapillary) and may lead toward architectural grading of lung adenocarcinoma (21,61,67).
The IASLC/ATS/ERS classification recognises four adenocarcinoma variants: invasive mucinous (formerly mucinous BAC), colloid, fetal (low or high grade) and enteric (21). Invasive mucinous adenocarcinomas have been classified as an adenocarcinoma variant, distinct from non-mucinous adenocarcinomas, due to the strong association of these tumours with KRAS mutations, lack of expression of TTF-1 and frequent multicentricity (21,61). Like their non-mucinous counterparts, mucinous adenocarcinomas can display varying amounts of lepidic, acinar, papillary or micropapillary architectural patterns with abundant mucin production (21,61).
Initial findings from a handful of studies employing the new IASLC/ATS/ERS classification indicate that the proposed histological subtypes may assist in the stratification and identification of patients at risk of poor clinical outcomes. As discussed previously, AIS and MIA have been associated with excellent prognosis (24-30,64,68-70). Intermediate prognosis is associated with the histological subtypes where papillary and acinar patterns predominate, whilst invasive mucinous or colloid variants or presence of predominant solid or micropapillary growth has been associated with poor prognosis (64,68,69).
Large cell carcinoma
LCC represents approximately 3% of all lung cancers (71-73) and is essentially a diagnosis of exclusion, where the tumour demonstrates no morphological features diagnostic of adenocarcinoma, SCC or SCLC (12,61). These tend to be large, partially necrotic tumours (Figure 5A) composed of sheets and nests of large polygonal cells with vesicular nuclei and prominent nucleoli (12) (Figure 5B). Although the current WHO classification is based purely on histological appearance, many of these undifferentiated tumours actually show evidence of glandular, squamous or NED when their ultrastructural (electron microscopy), immunophenotypic (IHC) or molecular features are examined (61). A recent review by Travis [2011] suggests that the diagnostic criteria for LCC should remain unchanged, but that pathology reports should comment if the tumour demonstrates evidence of squamous or adenocarcinomatous differentiation with modalities other routine histology (61).
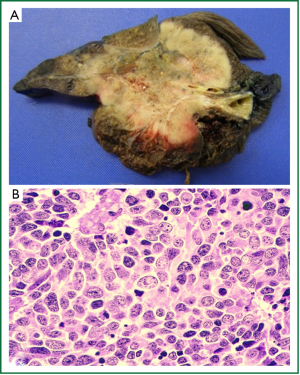
Since LCC is a diagnosis of exclusion, it can only be made on surgical resection specimens as histological assessment of the entire tumour is required to exclude focal differentiation. Therefore, a diagnosis of LCC cannot be made on small biopsies or cytology specimens, and in accordance with the new IASLC/ATS/ERS recommendations, these cases should be classified as NSCLC, not otherwise specified (discussed below) (21,61). Subtypes of LCC recognised by the 2004 WHO classification include large cell neuroendocrine carcinoma (LCNEC), basaloid carcinoma, lymphoepithelioma-like carcinoma, clear cell carcinoma and LCC with rhabdoid phenotype (12). LCNEC is discussed in further detail below.
Neuroendocrine tumours
Neuroendocrine tumours represent approximately 20-25% of all lung cancers (74,75) and form a subset of tumours with common morphological, molecular, immunohistochemical (IHC) and ultrastructural features that distinguish them from other lung tumours (12). The 2004 WHO classification separates neuroendocrine tumours of the lung into four categories: SCLC, LCNEC, typical carcinoid (TC) and atypical carcinoid (AC) tumours (12). Histologically, these tumours demonstrate varying degrees of neuroendocrine morphology, including organoid nesting, pallisading, trabecular growth and rosette-like structures. The major histological features differentiating the four types of neuroendocrine tumours are the presence or absence of necrosis and the mitotic rate (12).
Small cell lung carcinoma
SCLC is a highly aggressive neuroendocrine malignancy that accounts for approximately 12-14% of all lung cancers (71,76). The vast majority of patients have metastatic disease at the time of diagnosis, so surgical resection is rarely an option. Survival rates remain dismal, with only 5-8% of patients surviving 5 years after diagnosis (74,76).
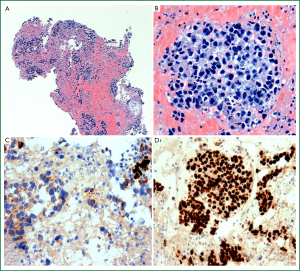
SCLC is an epithelial malignancy comprising small tumour cells (less than the diameter of 3 resting lymphocytes) with distinct cytological features including ill-defined cell borders, scant cytoplasm and finely granular nuclear chromatin without obvious nucleoli (Figure 6A; see also Table 1) (12). The presence of crush artefact (smearing of nuclear chromatin) and nuclear moulding are common but can be seen with other malignancies (e.g., crush artefact is common in lymphoid infiltrates). The mitotic rate is high (≥11 mitoses per 10 HPF) and there is often extensive necrosis (12). The distinctive histological appearance of SCLC allows for reliable diagnosis in small biopsy and cytology specimens, but for small specimens with significant crush artefact, use of a panel of IHC markers such as a pancytokeratin, neuroendocrine markers (chromogranin, synaptophysin and CD56) and/or TTF-1 and Ki-67 will confirm suspected SCLC (61,77) (Figure 6B-D). In cases where cytokeratin stains are negative, it is important to consider and exclude other diagnoses such as lymphoma, chronic inflammation, small round cell tumour or primitive neuroectodermal tumour (21, 61,78).
The 2004 WHO classification recognises two subtypes of SCLC: pure and combined (12). Combined small cell carcinoma is defined as a classical SCLC with a component showing features of any subtype of NSCLC, most often SCC, adenocarcinoma or LCC (12,61). A threshold for the proportion of non-small cell component is not required when this comprises adenocarcinoma or SCC, but for combined small cell and large cell tumours (SCLC-LC), at least 10% of the tumour must comprise a large cell component (12,61).
Large cell neuroendocrine carcinoma
LCNEC is another highly aggressive neuroendocrine malignancy, where tumour cells demonstrate cytological features of NSCLC (Table 1) but with neuroendocrine architecture (organoid nesting, pallisading, trabecular growth and rosette-like structures) and positive IHC stains for at least one neuroendocrine marker (chromogranin, synaptophysin or CD56) (12). Like SCLC, these tumours often show necrosis and have a high mitotic rate (≥11 mitoses per 10 HPF) (12). They may be pure LCNEC or combined with other types of NSCLC (12).
Making the diagnosis of LCNEC is often challenging due to significant overlap amongst the diagnostic groups (61,77). The differentiation between SCLC and LCNEC is particularly problematic, especially in cytology specimens, since there is overlap in nuclear size and some LCNEC do not contain prominent nucleoli. Currently there is no IHC stain for discrimination between SCLC and LCNEC and the distinction is based solely on cytological features (detailed in Table 1). Differentiating a LCNEC tumour from other NSCLC is based on the presence of neuroendocrine morphology and positive IHC for at least one neuroendocrine marker (12). However, up to 20% of NSCLC (adenocarcinoma, SCC and LCC) with no obvious neuroendocrine morphology demonstrate positive IHC staining for neuroendocrine markers (12,61). Currently, these tumours are classified as their NSCLC subtype but with neuroendocrine differentiation (NED) (i.e., NSCLC-NED) (12). The clinical significance of IHC evidence of NED without neuroendocrine morphology in NSCLC remains unclear and further research is required.
Carcinoid tumours
Carcinoid tumours comprise 1-2% of all lung tumours (74,75) and represent the most common lung tumour in children (79). Two subtypes of carcinoid tumour are recognised: typical carcinoid (TC) and AC (12). Both demonstrate morphological growth patterns indicative of NED (organoid, trabecular, insular, pallisading, ribbon, rosette-like structures) (12). The diagnostic criteria for differentiating TC and AC are mitotic rate and the presence or absence of necrosis. TC has <2 mitoses per 10 HPF and no necrosis. Conversely, AC show necrosis (usually focal or punctate) and/or 2-10 mitoses per 10 HPF (12).
Other NSCLC subtypes
Adenosquamous carcinoma
Adenosquamous carcinoma accounts for less than 5% of all lung cancers (80-82) and is defined as a NSCLC comprising at least 10% of both squamous and glandular differentiation (12). Similar to LCC, this diagnosis should be based on histology and not immunophenotype. However, further guidelines and definitions for characterisation using immunohistochemistry are likely in upcoming revisions of lung cancer classification. Adenosquamous carcinoma can only be diagnosed with certainty on surgical resections, however the diagnosis can be suspected in small biopsy or cytology specimens showing features of both squamous and glandular differentiation (21).
Sarcomatoid carcinoma
Sarcomatoid carcinoma is a poorly differentiated NSCLC that demonstrates morphological features of sarcoma or sarcoma-like (spindle and/or giant cells) differentiation, and represents approximately 1% of all lung cancers (12). The 2004 WHO classification recognises five subtypes, including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma and pulmonary blastoma (12). These highly aggressive tumours are believed to represent epithelial malignancies that have undergone divergent differentiation (12,83-87). Because of the heterogeneity of these tumours, they should not be diagnosed on small biopsy or cytology. Instead, the IASLC/ATS/ERS classification recommends using the diagnosis “poorly differentiated NSCLC with spindle and/or giant cell carcinoma” (21). Of note, the new IASLC/ATS/ERS classification now recognises fetal adenocarcinoma as a adenocarcinoma variant and not as an epithelial pattern of pulmonary blastoma (21,61,88).
Carcinomas of salivary gland type
Salivary gland tumours arising from bronchial glands are rare, representing less than 1% of all lung cancers (21). The 2004 WHO classification recognises three subtypes, including mucoepidermoid carcinoma, adenoid cystic carcinoma and epithelial-myoepithelial carcinoma (12).
Other primary tumours of the lung
As in any organ or tissue, primary tumours of the lung can arise from any cell type and are not purely derived from epithelial cells. Amongst the “non-epithelial” lung tumours, the 2004 WHO classification identifies broad groups including mesenchymal, lymphoproliferative and miscellaneous tumours (e.g., melanoma, germ cell tumours) (12). Detailed descriptions of these tumour types can be found in the 2004 WHO classification (12) and are beyond the scope of this review.
Diagnostic challenges
Patients with multiple lung tumours can present a diagnostic challenge for surgical pathologists. Discriminating between true independent primary lung cancers and a primary tumour with satellite lesions or intrapulmonary metastases is of critical importance, as the clinical management and prognosis varies significantly. Over 30 years ago, multiple primary lung cancers were defined as either synchronous (detected or diagnosed simultaneously) or metachronous (when there is a time interval between detection or diagnosis of two separate primary lesions) (89-91). To aid in diagnosis of metachronous tumours, the more common occurrence, the IASLC/ATS/ERS classification for adenocarcinomas notes the importance of not just reporting the predominant pattern of adenocarcinoma, but of detailed reporting of percentages of the various histological patterns in 5% increments (21). This allows for better comparison of subsequent adenocarcinomas, particularly if slides from the original primary tumour are not available for review (21).
Comprehensive histological and cytological examination of multiple tumours can distinguish primary from metastatic lung cancers in the majority of cases (92). But despite careful histological examination and IHC profiling, for a proportion of cases with multiple lung tumours, definitive distinction between multiple primary lung cancers and metastatic lung cancer may be impossible (89,90,93). Detailed clinical history and multidisciplinary case review is imperative to assist diagnosis. Genetic studies have demonstrated unique molecular phenotypes for multiple tumours with similar histology, suggesting that in the future molecular analysis of these tumours may provide greater diagnostic accuracy (90,91).
Differentiating metastases to the lung from primary lung cancers poses another diagnostic challenge for surgical pathologists, especially in small biopsy and cytology specimens. When initial histological examination of the specimen does not clearly indicate a primary lung malignancy, metastatic disease must be considered (61). Specific subtypes of lung cancer can be difficult to distinguish from metastatic disease, such as enteric differentiation in lung adenocarcinoma which shares morphologic and IHC features with colorectal adenocarcinoma (21,94). However, due to the heterogeneity of lung tumours, areas of more typical pulmonary differentiation should be evident (21). For example, lepidic growth favours primary adenocarcinoma (21) but rare cases of metastatic adenocarcinoma may show this pattern of spread (personal observation).
Differentiation of pulmonary SCC from metastatic head and neck SCC (HNSCC) presents a unique diagnostic challenge, as they demonstrate similar morphology and can occur in the same patient (95,96) due to similar aetiologies and risk factors (97,98). Recently, p16 has been investigated as a potential IHC marker for differentiating lung SCC from HNSCC with negative staining favouring lung SCC and positive staining favouring extrapulmonary SCC (99). However, a proportion of primary lung SCC stains positively for p16 (100) and may reflect the limited association of HPV infection with the development of lung cancer, with reports of HPV prevalence ranging from 5-22% (101,102). Currently, there is no reliable IHC marker for differentiating lung SCC from HNSCC (99,103,104). In cases where a metastatic lesion may be suspected, provision of detailed clinical history (i.e., history of previous malignancy and site) cannot be over emphasised, as it will guide detailed morphological and IHC assessment, and avoid wastage of valuable tissue on multiple IHC stains.
Small biopsies and cytology—the reality of non-small cell lung cancer diagnosis
The WHO classification of lung tumours was developed and designed for histological diagnosis and staging of surgically resected lung tumours. However, the vast majority of patients present with either locally advanced or metastatic disease and do not proceed to surgical resection, so the diagnosis of lung cancer is confirmed using small biopsies and/or cytology. With the advent of subtype-specific and targeted molecular therapies for lung cancer, the need for accurate histological classification and guided molecular characterisation is placing increasing demands on the surgical pathologist to do more with less tissue.
The recently published IASLC/ATS/ERS classification has, for the first time, provided a clinically focused and relevant classification applicable to small biopsies and cytology specimens. Of particular clinical importance is the necessity to differentiate between adenocarcinoma and SCC as this will guide subsequent molecular testing and therapeutic management. If a tumour demonstrates distinct histological features of adenocarcinoma or SCC then the standard diagnostic terms should be used (21,61). For poorly differentiated carcinomas, the IASLC/ATS/ERS advise the use of a limited IHC panel (discussed below) to differentiate adenocarcinoma and SCC, effectively reducing the use of the term NSCLC-NOS (not otherwise specified) whilst preserving tissue for molecular testing.
Of course, alternative diagnoses must be considered in the assessment of small biopsies/cytology specimens and provision of relevant clinical history from treating clinicians is essential in this process. Not only potentially benign conditions but other malignancies of the lung, both primary and metastatic, need to be considered. Both pathologists and treating clinicians need to be aware of inherent tumour heterogeneity and recognise that small biopsies/cytology specimens represent only a small sampling of the entire tumour; indeed, definitive diagnosis can only be made on surgical resection specimens for a subset of NSCLC (MIA, LCC, adenosquamous carcinoma). For small biopsy/cytology specimens, the IASLC/ATS/ERS suggests classification as NSCLC, with a description of the morphological features seen and whether a particular diagnosis is favoured, e.g., NSCLC with neuroendocrine morphology (positive neuroendocrine markers)—possible LCNEC (21).
Limiting use of NSCLC-NOS
No longer can tumours of the lung be simply classified as NSCLC or SCLC. There are now strong clinical indications driving the need for surgical pathologists to further subtype NSCLC, in particular to differentiate adenocarcinoma from SCC even on small biopsies/cytology specimens. In contrast to previous WHO classifications, the IASLC/ATS/ERS now recommend limiting use of the term NSCLC-NOS. All available clinical material must be utilised, and correlation carried out between cytology and histology specimens (21). Indeed, Sigel et al. [2011] reported a NSCLC-NOS diagnosis rate of 4% when cytology and small biopsies were correlated, reduced from 11% for cytology alone and 6% for biopsies alone (105).
For tumours in which differentiation is not evident on histological or cytological examination, a limited panel of histochemical and IHC markers is required (Figure 7A-D). The most widely used adenocarcinoma markers include mucin (periodic acid Schiff with diastase or mucicarmine), TTF-1 (thyroid transcription factor 1) and napsin-A, and for SCC the favoured markers are p63 and CK5/6. Of these, TTF-1 and p63 demonstrate the greatest sensitivity for their respective NSCLC subtypes (21,106-111). Alternative IHC markers such as CK7 (adenocarcinoma) and 34βE12 (squamous cell carcinoma) can be considered for indeterminate cases but have less sensitivity and specificity and tend not to be included in routine IHC panels (108,110-113). There has been recent interest in p40 (ΔNp63), a relatively new IHC marker for SCC. Current IHC stains for p63 detect all isoforms of the p63 gene, while p40 specifically detects non-transactivating or truncated forms of p63, resulting in increased specificity for SCC regardless of organ site (107,114-120).
Numerous diagnostic IHC algorithms for differentiating lung adenocarcinoma and SCC have been discussed in the literature, most including TTF-1 and p63 with or without a third or fourth stain. For example, Rekhtman and colleagues [2011] recently reported 100% accuracy in small biopsy specimens (diagnosis confirmed at surgical resection) with use of TTF-1 and p63 as a first line panel and addition of CK5/6 for equivocal cases (106). The IASLC/ATS/ERS advise the use of a single adenocarcinoma marker (TTF-1) and SCC marker (p63) with or without mucin stain, which should allow for differentiation of the majority of NSCLC (Table 2) (21,61). Where the IHC profile favours adenocarcinoma (TTF-1 positive and/or mucin positive, p63 negative), the tumour should be classified: “NSCLC, favour adenocarcinoma” (21,61). When the IHC profile favours SCC (p63 positive, TTF-1 negative, mucin negative), the tumour should be classified: “NSCLC, favour squamous cell carcinoma” (21,61). Only when there is no morphological or IHC evidence of clear lineage differentiation should the tumour be classified as NSCLC-NOS. In the hands of experienced pathologists and cytopathologists and judicial use of IHC stains, the IASLC/ATS/ERS estimate that less than 5% of NSCLC cases should be classified as NSCLC-NOS (21,61).
Molecular testing in NSCLC
The development of targeted molecular therapy for pulmonary adenocarcinoma has not only driven review of the classification and guidelines for NSCLC diagnosis, but also brought significant implications with regards to tissue sampling and processing. These increasingly small diagnostic biopsy and cytology specimens are no longer required purely for confirmation of malignancy and tumour subtyping, but there must be sufficient tumour tissue available for molecular testing to complete the pathological diagnostic assessment. Factors that need to be considered range from specimen collection, tissue processing in the laboratory, requests for molecular testing, provision of sufficient samples to the molecular laboratory and timely communication of results to the treating clinicians. Therefore the surgical pathologist must engage with the multidisciplinary team to develop strategic guidelines that will ensure that a complete histological and molecular diagnosis is provided for the patient (61).
In order to maximise diagnostic yield, it is crucial to optimise not only the amount but also the adequacy of the tissue sampled. Although the choice of procedure and sampling method will largely be guided by the lesion itself (i.e., size, location), patient factors (e.g., comorbidities) and available resources, pathologists should encourage collection of both cytology and biopsy specimens (if possible) as this can aid in diagnostic accuracy. Initially developed for non-ultrasound guided needle aspirates, rapid on site evaluation (ROSE) of specimen adequacy can be performed by trained cytopathologists and cytotechnologists, not only to confirm sampling of the target lesion but to ensure sufficient sample is collected (121,122). For various reasons, ROSE is not currently available in every institution (123). Interestingly, Alsharif et al. [2010] have shown that telepathology can be successfully used to assess adequacy of FNA (fine needle aspiration) specimens (120).
Once the specimen is collected, it is imperative that adequate clinical history is provided to ensure that the specimen is processed in a manner which will provide adequate diagnostic sections as well as preserving tissue for molecular testing. The IASLC/ATS/ERS guidelines strongly encourage surgical pathologists to minimise that amount of tissue used for diagnosis, in particular by limiting the number of first line IHC stains (discussed above) (21). Another suggested strategy is “reflex cutting” of paraffin blocks and preservation of unstained sections in order to avoid unnecessary loss of tissue during facing, although there is a small risk that DNA or epitope quality may degrade if not used shortly after sectioning (124,125).
Currently, activating mutations of EGFR are the best established and most widely used molecular biomarker in NSCLC. Additional molecular biomarkers are available, but with the exception of ALK translocations, are not recommended by the IASLC/CAP/AMP for routine testing (46). Fluorescence in situ hybridisation (FISH) remains the recommended method for clinical testing of ALK translocations, however the IASLC/CAP/AMP suggest that ALK IHC could be considered as a method for screening patients prior to formal ALK FISH testing (46).
Evolution of rapid and accurate EGFR mutation testing provides important data for clinical application. Traditionally the gold standard for EGFR mutation testing required direct sequencing of extracted tumour DNA, a time consuming methodology with low sensitivity (high levels of tumour DNA required). Newer validated methods for EGFR mutation testing provide increased sensitivity (fewer tumour cells required), improved turnaround times and allow for testing on a greater variety of clinical samples. Current techniques for EGFR mutation testing can be screening (detect all mutations including novel mutations, i.e., sequencing) or targeted methods (detect known mutations only). Of course, both approaches have their unique advantages and disadvantages (reviewed by Ellison et al., 2013) (126), and the available tests will vary amongst institutions. Therefore surgical pathologists must be aware of the available tests and specific tissue requirements for their local molecular laboratory.
Once a diagnosis of lung adenocarcinoma has been made, a decision must be made as to who is required to order the appropriate molecular testing. Implementation of “reflex” molecular testing initiated by the surgical pathologist (analogous to HER2 testing of invasive breast carcinoma) is a topic of debate and varies across institutions and government jurisdictions. The surgical pathologist may not be aware if the patient is a surgical candidate and that a more representative tissue specimen may follow. Conversely, delaying molecular testing may potentially contribute to delays in initiation of therapy. For patients diagnosed as NSCLC-NOS on small biopsy/cytology, the IASLC/ATS/ERS recommend biomarker testing (EGFR and ALK) (21) with discussion at multidisciplinary meetings to plan further testing and management.
Evolving role of the surgical pathologist
No longer is making a tissue diagnosis of pulmonary malignancy and the distinction between SCLC and NSCLC the only role of the surgical pathologist. Their contribution to lung cancer diagnosis, management and research is dynamic and continually evolving. The advent of personalised medicine for lung cancer has brought with it novel challenges and driven significant change. Of note is the first structured classification of lung cancer in small biopsy and cytology specimens developed by the IASLC/ATS/ERS, and a new classification of lung adenocarcinoma. Both have enhanced the clinical relevance of pathological diagnosis, allowing surgical pathologists to interact closely with clinicians to ensure that new concepts are understood and applied in the clinical setting.
With personalised medicine has come the development and clinical application of molecular testing in lung cancer. As the majority of patients never progress to surgical resection, the diagnostic small biopsy or cytology specimen has become a precious resource from which the surgical pathologist must aim to maximise diagnostic yield. Surgical pathologists have become the guardian for these limited precious samples, evaluating specimen adequacy, ensuring that appropriate processing techniques are applied, selecting suitable slides or blocks, enriching the tumour proportion by microdissection if required, and interpreting and providing timely results to the multidisciplinary team.
Furthermore, it is becoming increasingly important that surgical pathologists be involved in clinical trials and basic research to assist in the attainment of pathologically and clinically meaningful data. Where feasible, the surgical pathologist can also assist in the collection for research (with patient consent) tissue that is not required for clinical decision making. In this way, the modern surgical pathologist becomes an integral member of the multidisciplinary team, playing a crucial role in clinical trials and determining appropriate and timely management for patients with lung cancer.
Conclusions
During the last decade, through significant advances in our understanding of the complexity of lung tumour biology, we have finally entered the era of personalised medicine for lung cancer. No longer is basic tissue diagnosis and cancer staging alone central to determining treatment options for lung cancer, but histological subtyping and molecular testing have become of paramount importance. The surgical pathologist has become the guardian of the small biopsy/cytology specimen, a limited and precious resource from which diagnostic yield must be maximised.
Acknowledgements
Partial funding support for this work came from SPORE (P50CA70907) grant from the NCI, USA (AFG).
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Australian Bureau of Statistics. Causes of Death Australia 2009. Australian Government, ABS. 2011. Accessed 26 February 2012.
- Naruke T, Tsuchiya R, Kondo H, et al. Implications of staging in lung cancer. Chest 1997;112:242S-8S. [PubMed]
- Nesbitt JC, Putnam JB Jr, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg 1995;60:466-72. [PubMed]
- Australian Bureau of Statistics. Causes of Death, Australia, 2004. Australian Government, ABS. 2006. Accessed 2 October 2006.
- Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer 2004;101:3-27. [PubMed]
- Australian Institute of Health and Welfare (AIHW) Cancer Australia (CA) and Australian Association of Cancer Registries (AACR). Cancer survival and prevalence in Australia: cancers diagnosed from 1982 to 2004. Canberra: AIHW2008. Report No.: Cancer Series. No. 42: Cat. No. 38.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-67. [PubMed]
- Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science 2002;297:63-4. [PubMed]
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80. [PubMed]
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. WHO/IASLC classification of lung and pleural tumours. Lyon: IARC Press, 2004.
- Fong KM, Bowman RV, Fielding D, et al. Queensland integrated lung cancer outcomes project (qilcop): initial accrual and preliminary data from the first 30 months. Respirology 2003;8:A53.
- Kumar V, Abbas A, Aster J. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Elsevier/Saunders, 2013.
- Auerbach O, Forman JB, Gere JB, et al. Changes in the bronchial epithelium in relation to smoking and cancer of the lung; a report of progress. N Engl J Med 1957;256:97-104. [PubMed]
- Auerbach O, Stout AP, Hammond EC, et al. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med 1961;265:253-67. [PubMed]
- Zochbauer-Muller S, Gazdar AF, Minna JD. Molecular pathogenesis of lung cancer. Annu Rev Physiol 2002;64:681-708. [PubMed]
- Niklinski J, Niklinska W, Chyczewski L, et al. Molecular genetic abnormalities in premalignant lung lesions: biological and clinical implications. Eur J Cancer Prev 2001;10:213-26. [PubMed]
- Wistuba II, Gazdar AF. Characteristic genetic alterations in lung cancer. Methods Mol Med 2003;74:3-28. [PubMed]
- Wistuba II, Behrens C, Milchgrub S, et al. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene 1999;18:643-50. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Wistuba II, Gazdar AF. Lung cancer preneoplasia. Annu Rev Pathol 2006;1:331-48. [PubMed]
- Lantuéjoul S, Salameire D, Salon C, et al. Pulmonary preneoplasia--sequential molecular carcinogenetic events. Histopathology 2009;54:43-54. [PubMed]
- Sakurai H, Dobashi Y, Mizutani E, et al. Bronchioloalveolar carcinoma of the lung 3 centimeters or less in diameter: a prognostic assessment. Ann Thorac Surg 2004;78:1728-33. [PubMed]
- Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54. [PubMed]
- Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg 2001;71:971-4. [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [PubMed]
- Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg 2009;88:1106-11. [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [PubMed]
- Tomashefski JF Jr, Connors AF Jr, Rosenthal ES, et al. Peripheral vs central squamous cell carcinoma of the lung. A comparison of clinical features, histopathology, and survival. Arch Pathol Lab Med 1990;114:468-74. [PubMed]
- Davies SJ, Gosney JR, Hansell DM, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an under-recognised spectrum of disease. Thorax 2007;62:248-52. [PubMed]
- Wistuba II, Berry J, Behrens C, et al. Molecular changes in the bronchial epithelium of patients with small cell lung cancer. Clin Cancer Res 2000;6:2604-10. [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol 2008;3:111-6. [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [PubMed]
- Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 2006;118:257-62. [PubMed]
- Pham D, Kris MG, Riely GJ, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol 2006;24:1700-4. [PubMed]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [PubMed]
- Palmer RH, Vernersson E, Grabbe C, et al. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J 2009;420:345-61. [PubMed]
- Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol 2009;27:4232-5. [PubMed]
- Camidge DR, Kono SA, Flacco A, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res 2010;16:5581-90. [PubMed]
- Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011;6:459-65. [PubMed]
- Moreira AL, Thornton RH. Personalized medicine for non-small-cell lung cancer: implications of recent advances in tissue acquisition for molecular and histologic testing. Clinical lung cancer 2012;13:334-9. [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. [PubMed]
- Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev 2010;29:49-60. [PubMed]
- Roberts PJ, Stinchcombe TE, Der CJ, et al. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol 2010;28:4769-77. [PubMed]
- Vakiani E, Solit DB. KRAS and BRAF: drug targets and predictive biomarkers. J Pathol 2011;223:219-29. [PubMed]
- Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005;65:1642-6. [PubMed]
- Buttitta F, Barassi F, Fresu G, et al. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int J Cancer 2006;119:2586-91. [PubMed]
- Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010;2:62ra93. [PubMed]
- Monica V, Scagliotti GV, Ceppi P, et al. Differential Thymidylate Synthase Expression in Different Variants of Large-Cell Carcinoma of the Lung. Clin Cancer Res 2009;15:7547-52. [PubMed]
- Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006;107:1589-96. [PubMed]
- Nicolson MC, Fennell DA, Ferry D, et al. Thymidylate Synthase Expression and Outcome of Patients Receiving Pemetrexed for Advanced Nonsquamous Non-Small-Cell Lung Cancer in a Prospective Blinded Assessment Phase II Clinical Trial. J Thorac Oncol 2013;8:930-9. [PubMed]
- Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol 2009;27:4787-92. [PubMed]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Custodio AB, Gonzalez-Larriba JL, Bobokova J, et al. Prognostic and predictive markers of benefit from adjuvant chemotherapy in early-stage non-small cell lung cancer. J Thorac Oncol 2009;4:891-910. [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [PubMed]
- Maeshima AM, Maeshima A, Asamura H, et al. Histologic prognostic factors for small-sized squamous cell carcinomas of the peripheral lung. Lung Cancer 2006;52:53-8. [PubMed]
- Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669-92. [PubMed]
- Cagle PT, Allen TC, Dacic S, et al. Revolution in lung cancer: new challenges for the surgical pathologist. Arch Pathol Lab Med 2011;135:110-6. [PubMed]
- Charloux A, Quoix E, Wolkove N, et al. The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol 1997;26:14-23. [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [PubMed]
- Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101-9. [PubMed]
- Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol 2007;20:638-47. [PubMed]
- Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010;34:1155-62. [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [PubMed]
- Howlader N NA, Krapcho M, Garshell J, et al. eds. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, , based on November 2012 SEER data submission, posted to the SEER web site, April 2013.
- Sun Z, Aubry MC, Deschamps C, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg 2006;131:1014-20. [PubMed]
- Sawabata N, Asamura H, Goya T, et al. Japanese Lung Cancer Registry Study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol 2010;5:1369-75. [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [PubMed]
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38. [PubMed]
- Australia AIoHaWC. Lung cancer in Australia: an overview. Canberra: AIHW2011. Report No.: Cancer series no. 64. Cat. no. CAN 58.
- Fan Z, Schraeder R. The changing pathology of lung cancer. Surg Oncol Clin N Am 2011;20:637-53. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of Lung Cancer in Small Biopsies and Cytology: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification. Arch Pathol Lab Med 2013;137:668-84. [PubMed]
- Lack EE, Harris GB, Eraklis AJ, et al. Primary bronchial tumors in childhood. A clinicopathologic study of six cases. Cancer 1983;51:492-7. [PubMed]
- Fitzgibbons PL, Kern WH. Adenosquamous carcinoma of the lung: a clinical and pathologic study of seven cases. Hum Pathol 1985;16:463-6. [PubMed]
- Naunheim KS, Taylor JR, Skosey C, et al. Adenosquamous lung carcinoma: clinical characteristics, treatment, and prognosis. Ann Thorac Surg 1987;44:462-6. [PubMed]
- Ben Y, Yu H, Wang Z, et al. Adenosquamous lung carcinoma: clinical characteristics, surgical treament and prognosis. Chin Med Sci J 2000;15:238-40. [PubMed]
- Dacic S, Finkelstein SD, Sasatomi E, et al. Molecular pathogenesis of pulmonary carcinosarcoma as determined by microdissection-based allelotyping. Am J Surg Pathol 2002;26:510-6. [PubMed]
- Nappi O, Glasner SD, Swanson PE, et al. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of ‘carcinosarcomas’ and ‘spindle-cell carcinomas’. Am J Clin Pathol 1994;102:331-40. [PubMed]
- Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol 1996;20:277-85. [PubMed]
- Nakajima M, Kasai T, Hashimoto H, et al. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer 1999;86:608-16. [PubMed]
- Ro JY, Chen JL, Lee JS, et al. Sarcomatoid carcinoma of the lung. Immunohistochemical and ultrastructural studies of 14 cases. Cancer 1992;69:376-86. [PubMed]
- Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med 2010;134:1645-58. [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Huang J, Behrens C, Wistuba I, et al. Molecular analysis of synchronous and metachronous tumors of the lung: impact on management and prognosis. Ann Diagn Pathol 2001;5:321-9. [PubMed]
- Hiroshima K, Toyozaki T, Kohno H, et al. Synchronous and metachronous lung carcinomas: molecular evidence for multicentricity. Pathol Int 1998;48:869-76. [PubMed]
- Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 2009;33:1752-64. [PubMed]
- Neugut AI, Sherr D, Robinson E, et al. Differences in histology between first and second primary lung cancer. Cancer Epidemiol Biomarkers Prev 1992;1:109-12. [PubMed]
- Inamura K, Satoh Y, Okumura S, et al. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol 2005;29:660-5. [PubMed]
- Leong PP, Rezai B, Koch WM, et al. Distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. J Natl Cancer Inst 1998;90:972-7. [PubMed]
- Geurts TW, Nederlof PM, van den Brekel MW, et al. Pulmonary squamous cell carcinoma following head and neck squamous cell carcinoma: metastasis or second primary? Clin Cancer Res 2005;11:6608-14. [PubMed]
- Day TA, Davis BK, Gillespie MB, et al. Oral cancer treatment. Curr Treat Options Oncol 2003;4:27-41. [PubMed]
- Keith RL, Miller YE. Lung cancer: genetics of risk and advances in chemoprevention. Curr Opin Pulm Med 2005;11:265-71. [PubMed]
- Pereira TC, Share SM, Magalhaes AV, et al. Can we tell the site of origin of metastatic squamous cell carcinoma? An immunohistochemical tissue microarray study of 194 cases. Appl Immunohistochem Mol Morphol 2011;19:10-4. [PubMed]
- Doxtader EE, Katzenstein AL. The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: a study of 137 cases. Hum Pathol 2012;43:327-32. [PubMed]
- Fong KM, Kida Y, Zimmerman PV, et al. Loss of heterozygosity frequently affects chromosome 17q in non-small cell lung cancer. Cancer Res 1995;55:4268-72. [PubMed]
- Klein F, Amin Kotb WF, Petersen I. Incidence of human papilloma virus in lung cancer. Lung Cancer 2009;65:13-8. [PubMed]
- Suo Z, Holm R, Nesland JM. Squamous cell carcinomas. An immunohistochemical study of cytokeratins and involucrin in primary and metastatic tumours. Histopathology 1993;23:45-54. [PubMed]
- Kaufmann O, Fietze E, Mengs J, et al. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol 2001;116:823-30. [PubMed]
- Sigel CS, Moreira AL, Travis WD, et al. Subtyping of non-small cell lung carcinoma: a comparison of small biopsy and cytology specimens. J Thorac Oncol 2011;6:1849-56. [PubMed]
- Rekhtman N, Ang DC, Sima CS, et al. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol 2011;24:1348-59. [PubMed]
- Righi L, Graziano P, Fornari A, et al. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology: a retrospective study of 103 cases with surgical correlation. Cancer 2011;117:3416-23. [PubMed]
- Mukhopadhyay S. Utility of small biopsies for diagnosis of lung nodules: doing more with less. Mod Pathol 2012;25:S43-57. [PubMed]
- Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol 2010;41:20-5. [PubMed]
- Nicholson AG, Gonzalez D, Shah P, et al. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol 2010;5:436-41. [PubMed]
- Loo PS, Thomas SC, Nicolson MC, et al. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol 2010;5:442-7. [PubMed]
- Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am J Surg Pathol 2011;35:15-25. [PubMed]
- Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 2000;13:962-72. [PubMed]
- Pelosi G, Rossi G, Cavazza A, et al. ΔNp63 (p40) Distribution Inside Lung Cancer: A Driver Biomarker Approach to Tumor Characterization. Int J Surg Pathol 2013;21:229-39. [PubMed]
- Candi E, Dinsdale D, Rufini A, et al. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle 2007;6:274-85. [PubMed]
- Bishop JA, Teruya-Feldstein J, Westra WH, et al. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol 2012;25:405-15. [PubMed]
- Pelosi G, Fabbri A, Bianchi F, et al. ΔNp63 (p40) and thyroid transcription factor-1 immunoreactivity on small biopsies or cellblocks for typing non-small cell lung cancer: a novel two-hit, sparing-material approach. J Thorac Oncol 2012;7:281-90. [PubMed]
- Yang X, Lu H, Yan B, et al. ΔNp63 versatilely regulates a Broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res 2011;71:3688-700. [PubMed]
- Saintigny P, El-Naggar AK, Papadimitrakopoulou V, et al. DeltaNp63 overexpression, alone and in combination with other biomarkers, predicts the development of oral cancer in patients with leukoplakia. Clin Cancer Res 2009;15:6284-91. [PubMed]
- Alsharif M, Carlo-Demovich J, Massey C, et al. Telecytopathology for immediate evaluation of fine-needle aspiration specimens. Cancer Cytopathol 2010;118:119-26. [PubMed]
- Bulman W, Saqi A, Powell CA. Acquisition and processing of endobronchial ultrasound-guided transbronchial needle aspiration specimens in the era of targeted lung cancer chemotherapy. Am J Respir Crit Care Med 2012;185:606-11. [PubMed]
- Wallace WA, Rassl DM. Accuracy of cell typing in nonsmall cell lung cancer by EBUS/EUS-FNA cytological samples. Eur Respir J 2011;38:911-7. [PubMed]
- Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer 2001;93:319-22. [PubMed]
- Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med 2011;32:22-31. [PubMed]
- Thunnissen E, Kerr KM, Herth FJ, et al. The challenge of NSCLC diagnosis and predictive analysis on small samples. Practical approach of a working group. Lung cancer 2012;76:1-18. [PubMed]
- Ellison G, Zhu G, Moulis A, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol 2013;66:79-89. [PubMed]




