Non-intubated single-incision video-assisted thoracic surgery: a two-center cohort of 188 patients
Introduction
Non-intubated video-assisted thoracic surgery (NI-VATS) has evolved significantly in the last decade (1-3). Thoracic surgeons seek for a less invasive approach and developed single-incision VATS (also called uniportal VATS) and modifications, trying to decrease postoperative pain compared to multiportal VATS. Certainly, not only surgical invasiveness but also trying to decrease anesthetic aggression by avoiding orotracheal intubation and mechanical ventilation while preserving spontaneous breathing may potentially benefit and shorten the postoperative course. In a comparative study of non-intubated versus intubated VATS segmentectomies, an earlier resumption of oral intake, less cost of anesthesia, shorter postoperative chest tube duration and a trend towards less postoperative stay and complications were observed in the NI-VATS cohort (4), and a more recent propensity score matching analysis in 2016 showed significant shorter fasting time and hospital stay (5). In addition, some high-risk patients are excluded for thoracic surgery due to functional limitation, but experience has been acquired with awake non-intubated procedures in severe emphysematous patients for lung-volume reduction surgery that allows these compromised patients to be performed pulmonary resections (6,7). In a step-forward to a less invasive approach, non-intubated single-incision procedures [non-intubated single-incision video-assisted thoracic surgery (NI-SI-VATS)] are slowly expanding, mainly in Asia and Europe (8-12). There is still a lack of comparative and multicenter studies, therefore the evidence is limited when comparing to multiportal VATS under spontaneous breathing and standard intubated-ventilated procedures.
The largest comparative study between epidural anesthesia and intercostal blockade for non-intubated procedures showed a significant reduction of anesthesia induction time and surgery, less need of vasoactive drugs and blood loss, and also a trend towards less chest tube duration in the intercostal blockade group (1).
The aim of this study is to describe the clinicopathological features of the patients and compare the outcomes of two different anaesthetic protocols of single-incision procedures under spontaneous breathing (NI-SI-VATS) in two different countries.
Methods
Study design
We present a retrospective series of the patients undergoing NI-SI-VATS operations in two centers: National Taiwan University Hospital (NTUH, Taiwan) and Hospital General Universitario Alicante (HGUA, Spain). This study was approved by the Research Ethics Committee of both centers (Ref numbers: 201604079RINB and PI2015/67).
Medical records were reviewed retrospectively between July 2013 and November 2015. Four surgeons in NTUH and two surgeons in HGUA performed non-intubated procedures. Patients who met the inclusion criteria and did not present any exclusion criteria were candidates for NI-SI-VATS. The operations were performed under standard single-incision VATS approach. Only when the incision was performed under this non-intubated approach, the procedure was considered and included in the study.
Patient selection
Patients over 18 years old with thoracic lesions requiring VATS and with American Society of Anesthesiologists Physical Status Classification (ASA) less or equal than III were initially included.
For undetermined pulmonary nodules, which primary lung cancer cannot be excluded, only peripheral and small lesions (<2 cm) with ground-glass opacity (GGO)-dominant (50% or more GGO) pattern would be considered for NI-SI-VATS. In patients with such lesions, preoperative CT-guided dye localization and intraoperative frozen section were routinely performed to ensure satisfactory oncological treatment (13,14).
Patients were excluded if they presented evidence of abundant pleural adhesions or major organ involvement on chest computed tomography (CT), anticipated difficulty in airway management, chest deformity, morbid obesity with body mass index (BMI) superior to 35, coagulopathy or predicted postoperative forced expiratory volume in first second (ppoFEV1) less than 30%.
Signed informed consent for the study and exhaustive explanation was mandatory in all patients (15).
Anesthetic management
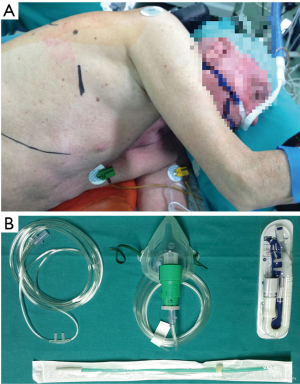
Center A (NTUH): Patients were premedicated with 50 to 100 µg of intravenous fentanyl. The respiratory rate and end-tidal carbon dioxide (ETCO2) were continuously monitored by insertion of a detector into one nostril. A BISTM Quatro bispectral index sensor was routinely applied to monitor the level of consciousness. The patients were sedated with intravenous propofol using a target-controlled infusion (BIS value between 40 and 60). Incremental intravenous injections of fentanyl 25 to 50 µg were given as needed. The patients breathed oxygen spontaneously through ventilation mask in the lateral decubitus position (Figure 1). After applying local anesthesia with 2% lidocaine in fifth or sixth intercostal space, a 3-cm incision was made. An iatrogenic pneumothorax was created and the lung collapsed gradually while the patient remained spontaneously breathing. The incision was kept open by a wound protector retractor. Under guidance of a 5-mm thoracoscope, intrathoracic intercostal nerve blocks were established by infiltrating 0.5% bupivacaine (1.5 mL in each intercostal space) from the third to the eighth intercostal nerve under the parietal pleura, 2 cm lateral to the sympathetic chain, using a 25-G top-winged infusion needle. A vagal nerve block was performed by infiltration of 3 mL of 0.5% bupivacaine at the level of the lower trachea for right-sided procedures, and the level of the aortopulmonary window for left-sided procedures, to inhibit the cough reflex.

Center B (HGUA): Patients were operated under awake regimen through conscious sedation (Ramsay sedation scale 1–2) with remifentanil infusion 0.05–0.1 µg/kg/min and intravenous bolus of 1–2 mg of midazolam (11). Sensitive and motor block between T2 and T9 were achieved through epidural catheter between T4–5, and 4–5 mL consecutive bolus of ropivacaine 0.375% infusion. During the operation, patients were placed in the lateral decubitus position; high-flow oxygen nasal prongs at 40 L/minute (lpm), and non-invasive ETCO2 and invasive radial artery monitoring to control oxygen saturation and avoid excessive hypercapnia (Figure 2). Routine monitoring was done as previously described. The incision was covered by a wound protector retractor. An iatrogenic pneumothorax was created and the lung collapsed while the patient remained spontaneously breathing. In patients presenting cough reflex and in major procedures, a vagal nerve block was also performed.
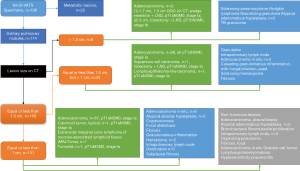
Criteria for conversion to multiport VATS or tracheal intubation
Conversion to multiport VATS included extensive pleural adhesions not anticipated on CT scan, unfavorable anatomic structures that hinder dissection, inadequate collapse, or mild to moderate bleeding. The decision to perform tracheal intubation was made based on the development of respiratory acidosis with respiratory rate above 30 bpm, refractory hypoxemia (oxygen saturation under 90% or mean arterial oxygen pressure under 60 mmHg), deep respiratory movements, unstable hemodynamic status, ineffective vagal block with persistent cough, panic attack, voluntary desire of the patient, or severe bleeding requiring thoracotomy (15-17). The surgeon and the anesthesiologist made the decision together through the emergency protocols already described (10,15).
Postoperative care
After the operation, the patients were able to resume water and food intake within 2 hours. Paracetamol, oral nonsteroidal analgesics (NSAIDs) and tramadol were used for postoperative pain control. Patient-controlled analgesia with intravenous morphine (1 mg/mL) was provided if the patient requested additional analgesia. Postoperative pain intensity was evaluated on postoperative day (POD) 1 and 2 using a numeric pain intensity scale (Visual Analogue Scale, VAS). A chest X-ray was obtained within the first 24 hours, and then daily. The chest tube was removed if no air leak was detected and the fluid drained less than 200–250 mL per day. Prolonged air leakage was defined as persistent air leak for 3 or more days after the operation (10). All postoperative complications were recorded.
Data collection and analysis
Demographic data, clinicopathological features, preoperative tests, and final outcomes were retrospectively collected in a database. Statistical analysis was performed using STATA 14.0. Findings are expressed as means ± standard deviation or median (range) unless otherwise specified. Results were considered statistically significant for P values <0.05. Findings based on nominal data were tested using the Fisher’s exact test; otherwise, parametric independent Student t tests and one-way analysis of variance (ANOVA) were used.
Results
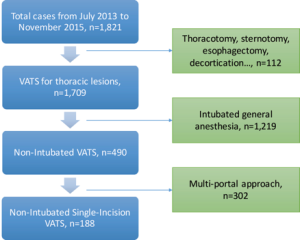
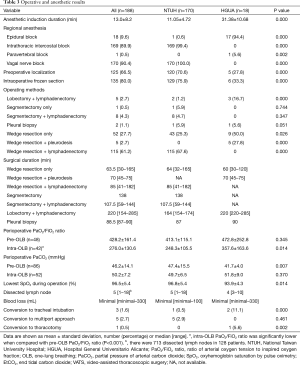
Between July 2013 and November 2015, there were 1,709 patients undergoing surgical management for thoracic diseases (Figure 3). Among them, 1,006 patients were managed by VATS (18). According to the patient selection protocol, a total of 188 patients were operated under NI-SI-VATS (170, 90.4% in NTUH; 18, 9.6% in HGUA), with a mean age of 56 (56±12.5) years old without differences between centers. Most patients were women (147, 78.2%), with significant differences between NTUH and HGUA (83.5% vs. 27.8% respectively). Mean BMI was 22.7±3.3 (22.5±2.8 in NTUH patients; 24.8±6.1 in HGUA patients, P=0.005), and only 13.3% of the patients had a history of smoking (5.3% of NTUH patients; 77.8% of HGUA patients, P<0.001). Preoperative lung function was optimal in most patients [predicted postoperative forced vital capacity (ppoFVC) 104.9%±17.9%; ppoFEV1 104.2%±21.2%; FEV1/FVC 83.4%±6.2%)] with more compromised patients in the HGUA cohort (P=0.005, 0.003, 0.018, respectively). Clinical characteristics are shown in Table 1.
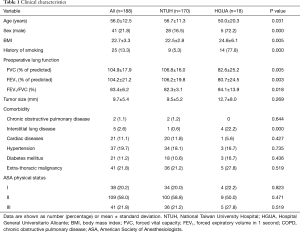
Full table
The procedure most frequently performed was wedge resection in 172 patients (91.4%) and anatomical resections were done in 14 (5 lobectomies and 9 anatomical segmentectomies), being more frequent in HGUA center (16.6%; 3 of 18 cases) than in NTUH Center (6.4%, 11 of 170 cases).
Global mean anesthesia induction time was 13±8.2 minutes, being higher in the HGUA center in which epidural block was routinely performed (31.38±10.68 vs. 11.05±4.72; P<0.001). Most used regional anesthesia technique was intrathoracic intercostal block (169 patients, 89.9%) which was dominantly performed in the NTUH cohort. Vagal nerve block was done in 170 patients (90.4%). Pre-one-lung breathing (Pre-OLB) mean PaCO2 was 46.2 mmHg, while intra-OLB mean PaCO2 was 50.2 mmHg, not reaching significance. Mean lowest oxygen saturation was 96.5%.
The operation time varied: between 62 to 89 minutes for wedge resection; 106 minutes for anatomical segmentectomies; finally, the most lasting procedures were lobectomies with 210 minutes including lymphadenectomy. Intraoperative frozen section examination was done in 135 cases (72%).
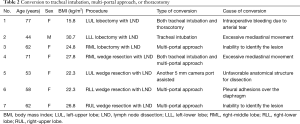
There were 3 conversions to tracheal intubation (1.6%), and all were performed in the lateral decubitus position successfully (Table 2). Among them, 2 cases were due to excessive mediastinal movement and one related to a lingular arterial tear with bleeding, which also needed conversion to thoracotomy. In this case, bleeding was controlled by compression with a sponge stick, and then, following the Emergency Protocol, conversion to general anesthesia and orotracheal selective intubation in lateral decubitus was accomplished in 8 minutes while thoracoscopy evidenced successful control of the bleeding. Conversion to open thoracotomy was accomplished in 5 minutes as direct repair was not feasible by SI-VATS, but successful through the open approach. Median blood loss was minimal with the highest loss in the case of arterial tear with 330 mL. Five cases required additional ports (2.7%) in the NTUH cohort: 2 cases due to inability to palpate the lesion, 1 case due to excessive mediastinal movement, 1 related to excessive pleural adhesions to diaphragm and 1 related to the need of assistance (Table 2). Operative and anesthetic results are shown in Table 3.
Full table
Full table
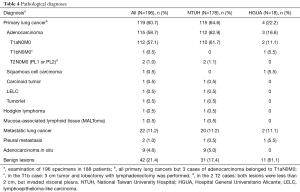
Primary lung cancer was the most frequent pathological diagnosis (119, 60.7%) with high predominance of adenocarcinoma (115, 96.6%). In patients who had non-small cell lung cancer, pathological staging was T1N0M0 in 116 patients (97%), T1bN0M0 in 1 patient, and T2N0M0 in the remaining 2 patients because of visceral pleura invasion (Table 4). Mean tumor size was 9.5 mm. Benign lesions were the second most frequent diagnosis with 42 cases (21.4%), and metastatic disease in 22 cases (11%). Preoperative CT-guided localization was performed in 125 patients (66.5%), with a mean small tumor size of 9.5 mm.
Full table
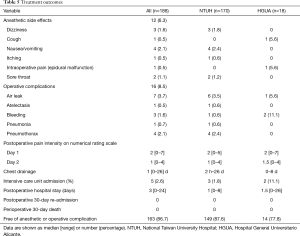
Anesthetic side effects weren’t common (6.3%) and mainly related to nausea/vomiting (2.1%), without differences. Postoperative complication rate was 8.5% (7.6% in NTUH; 16.6% in HGUA ; P=0.192), being air leak the most frequent in 7 cases (3.7%). One-hundred and sixty-three patients (86.7%) remained without postoperative complication.
Mean pain VAS score was 2 in POD1, and 1.4 in POD2. Median chest drainage duration was 1 day (0–26 days), and median postoperative hospital stay was 3 days (0–24 days) (Table 5).
Full table
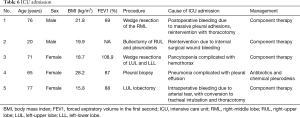
Five patients required ICU admission (2.6%): one intraoperative bleeding requiring thoracotomy, three patients with postoperative bleeding (pleural adhesions, surgical wound bleeding and pancytopenia) and one patient due to pneumonia and pleural effusion (Table 6). There was no in-hospital death, neither 30-day readmission nor 30-day death.
Full table
Discussion
Non-intubated procedures have been progressively reintroduced in the clinical practice and proved feasible and safe (1,10,15,19-23). Combination of non-intubated surgery under SI-VATS for pulmonary resections was first described by Rocco in 2010 (8), and then some series have been published (1,10). This is the first retrospective series of NI-SI-VATS in two different centers comparing two different anesthetic regimens.
Predominance of women (78.2%) was mainly related to their greater proportion in the NTUH cohort, where non-smoking women had been found to contribute to rising portion of primary lung cancer. BMI is important because non-intubated procedures should not be attempted in obese patients (24), regarding the anatomical disadvantage of a higher mediastinal to chest ratio, the higher diaphragm and potential respiratory depression. The healthy nature of our cohort is related to the high prevalence of non-smoking lung cancer female thin patients without severe respiratory comorbidity in the Asian cohort.
Malignant subcentimetric tumors were the most frequent diagnosis and wedge resection the most common procedure. The mean tumor size in this highly-selective study is 9.7 mm with mainly GGO-dominant lesions, especially in the NTUH cohort. We only selected sub-centimeter GGO-dominant lesions in which primary lung cancer cannot be excluded for non-intubated single-incision wedge resection as surgical management both for diagnosis and treatment (14). Intraoperative frozen section was routinely performed to ensure the resection margins to be more than 2 cm if it showed primary lung cancer. Additional resection was occasionally needed if section margin were less than 2 cm. Meanwhile, lymph node dissection (LND) was performed to complete the pathological staging. There were 2 primary lung cancer >1.5 cm: one 1.7 cm adenocarcinoma with preoperative 1.5 cm GGO pattern on CT, where a wedge resection with LND was performed; and the other 3.0 cm adenocarcinoma where lobectomy with LND was performed (Figure 4). Our series also included 14 anatomical segmentectomies and lobectomies, mainly performed for primary lung cancer. Discrepancy in the volume between both centers reflects anatomical differences (Asian population thinner than European), differences in surgical weekly work-load, and that in HGUA only two surgeons performed these procedures and there were no departmental criteria for selecting these “ideal-patients” for non-intubated.
Intrathoracic intercostal and epidural blockade proved to be safe, with a low rate of side effects (global 6.3%; intercostal 6.4%; epidural 5.5%). Intraoperative pain due to epidural malfunction was very low, just reported in only 1 case (0.5%), and is explained by the high experience of anesthesiologists of HGUA who are highly-specialized in thoracic anesthesia. Although not systematically performed in many centers, epidural anesthesia was considered traditionally the gold-standard in the HGUA without the need of guidance. Mean anesthesia induction time of 13 minutes seems reasonable in order to develop fast-track protocols. Intercostal blockade had a significant shortening of induction time when comparing to epidural blockade (11 vs. 31 minutes) with the advantage of the thoracoscopic guidance and unilateral effect. Low pain scale scores at POD1 and POD2 indicate that single-incision VATS is a scarcely painful approach (POD1 1.98 intercostal vs. 2.05 epidural; POD2 1.34 intercostal vs. 1.62 epidural; P=0.856, 0.369, respectively) (25).
Operative complication rate is also low (8.5%) and usually related to air leak and residual pneumothorax. Postoperative bleeding occurred only in 3 cases (1.6%): one related to massive pleural adhesions, the second related to intercostals muscle intrathoracic bleeding and the last one related to transient myelosuppression with pancytopenia.
With less invasiveness and less need for analgesia, oral intake within the first 2 hours and patient mobilization could be resumed soon. Median chest drainage of 1 day and median postoperative stay of 3 days are similar to those results reported in most of the VATS studies, but we hypothesized that NI-SI-VATS surgery could potentially be proposed as a fast track protocol. No 30-day readmission and no death indicates that NI-SI-VATS can be considered safe for selected patients in experienced centers.
During the setting of a non-intubated program, emergency protocols should be carefully developed. The conversion to tracheal intubation was very low (1.6%), and of the 3 cases, two were elective and not emergent, while the third one required and emergent conversion to intubation and thoracotomy due to a vascular injury controlled by compression and with only a moderate blood loss. Anesthesiologists in both centers had previously developed a learning curve of selective intubation in the lateral decubitus position before starting these procedures. Conversion to multiport VATS was also infrequent (2.7%) and not emergent. Surgeons and anesthesiologists that embark in a non-intubated program should begin with minor procedures in strictly selected patients, in a cooperative environment and after a comprehensive process of understanding non-intubated changes in respiratory physiology, potential complications, and how to manage any emergent situation. There is still a lack of accreditation in non-intubated programs, as the Non-Intubated Thoracic Surgery Working Group (European Society of Thoracic Surgeons) declared in 2015 (26).
Main objective advantages of non-intubated procedures are the earlier resumption of oral intake, shorter postoperative duration of chest tube, shorter postoperative hospital stay and a trend towards less complications (4,5), but there are very few comparative studies and a clear lack of evidence to support one against the other. A recent meta-analysis of 2016 including 1283 patients showed shorter in-operating room time, shorter hospital stay and a decrease in postoperative complications when compared to intubated procedures (27). Although controversial outcomes have been published, the most recent comparative analysis in terms of postoperative pain reported significant less postoperative pain and improved short-term postoperative quality of life in a uniportal cohort of 115 patients versus a 3-port cohort of 101 patients (28). Thus we hypothesized that NI-SI-VATS could potentially offer a beneficial and safe profile for selected patients, because there is evidence that patients that not fit the strict selection criteria may develop complications (29) during the procedure such as excessive hypercapnia, hypoxemia, excessive mediastinal and diaphragmatic movement with potential complications during the technical steps of the surgery (such as a tear in pulmonary artery or vein branches). So we think that NI-SI-VATS could be offered to selected patients in order to offer a faster postoperative recovery time and a safe procedure.
Main limitations of this study are its retrospective character, the small sample size in one of the arms, the differences between the anesthetic management between the two centers and the lack of a control group (intubated, multiport VATS), and also the selection bias: only strictly selected patients are considered nowadays for non-intubated surgery. Although the Spanish center represents less than 10% of total cases, it is one of the first series of NI-SI-VATS published in European population. It would be recommended to increase the sample size of the Spanish center to compare these two different anaesthetic techniques. However, the results are encouraging and impels us to keep on investigating.
Conclusions
Our results of NI-SI-VATS suggest that it may be a feasible and safe approach, even for pulmonary anatomical resections. Strict selection criteria, surgical skills, experience of both NI-VATS and SI-VATS are mandatory requirements for initiating a NI-SI-VATS program. Comparative cohorts and randomized trials for intubated and multiport VATS should be encouraged to improve the validity of the results and to seek for differences in morbidity, postoperative course and safety.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Research Ethics Committee of both centers (Ref numbers: 201604079RINB and PI2015/67). Signed informed consent for the study and exhaustive explanation was mandatory in all patients.
References
- Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: a retrospective cohort study of 238 cases. Medicine (Baltimore) 2015;94:e727. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, Rodriguez JL, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Guo Z, Yin W, Pan H, et al. Video-assisted thoracoscopic surgery segmentectomy by non-intubated or intubated anesthesia: a comparative analysis of short-term outcome. J Thorac Dis 2016;8:359-68. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo TC. Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia. Eur J Cardiothorac Surg 2011;39:e51-8. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Awake Thoracic Surgery Research Group. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg 2010;89:1625-7. [Crossref] [PubMed]
- Li S, Cui F, Liu J, et al. Nonintubated uniportal video-assisted thoracoscopic surgery for primary spontaneous pneumothorax. Chin J Cancer Res 2015;27:197-202. [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostals nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Galvez C, Bolufer S, Navarro-Martinez J, et al. Awake uniportal video-assisted thoracoscopic metastasectomy after a nasopharyngeal carcinoma. J Thorac Cardiovasc Surg 2014;147:e24-6. [Crossref] [PubMed]
- Rocco G. Non-intubated uniportal lung surgery. Eur J Cardiothorac Surg 2016;49 Suppl 1:i3-i5. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-544.e2. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Navarro-Martínez J, Gálvez C, Rivera-Cogollos MJ, et al. Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery. Ann Transl Med 2015;3:111. [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [Crossref] [PubMed]
- Galvez C, Bolufer S, Navarro-Martinez J, et al. Non-intubated video-assisted thoracic surgery management of secondary spontaneous pneumothorax. Ann Transl Med 2015;3:104. [PubMed]
- Pompeo E, Rogliani P, Palombi L, et al. Awake Thoracic Surgery Research Group (ATSRG). The complex care of severe emphysema: role of awake lung volume reduction surgery. Ann Transl Med 2015;3:108. [PubMed]
- Matsumoto I, Oda M, Watanabe G. Awake endoscopic thymectomy via an infrasternal approach using sternal lifting. Thorac Cardiovasc Surg 2008;56:311-3. [Crossref] [PubMed]
- Peng G, Cui F, Ang KL, et al. Non-intubated combined with video-assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. [Crossref] [PubMed]
- Chen J, Lin J, Tu Y, et al. Nonintubated Transareolar Endoscopic Thoracic Sympathectomy with a Flexible Endoscope: Experience of 58 Cases. Ann Thorac Cardiovasc Surg 2016;22:12-9. [Crossref] [PubMed]
- Kiss G, Castillo M. Nonintubated anesthesia in thoracic surgery: general issues. Ann Transl Med 2015;3:110. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Pompeo E, Sorge R, Akopov A, et al. ESTS Non-intubated Thoracic Surgery Working Group. Non-intubated thoracic surgery-A survey from the European Society of Thoracic Surgeons. Ann Transl Med 2015;3:37. [PubMed]
- Deng HY, Zhu ZJ, Wang YC, et al. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:31-40. [Crossref] [PubMed]
- Hao Z, Cai Y, Fu S, et al. Comparison Study of Post-operative Pain and Short-term Quality of Life between Uniportal and Three Portal Video-assisted Thoracic Surgery for Radical Lung Cancer Resection. Zhongguo Fei Ai Za Zhi 2016;19:122-8. [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery Ann Transl Med 2014;2:106. [PubMed]