Anti-cancer activity of dose-fractioned mPE +/− bevacizumab regimen is paralleled by immune-modulation in advanced squamous NSLC patients
Introduction
Non-small cell lung cancer (NSCLC) is the most common malignancy and the leading cause of cancer death representing about 17% of new cancer diagnoses worldwide (1,2). Squamous-NSCLC (sqNSCLC) (3) represents the second most common histology (30% of all cases), and for these patients the only chance of cure is represented by radical surgery that is possible only in case of loco-regional disease (stage I–IIIA) and good performance status. The standard treatment for advanced sqNSCLC patients (stage IIIB–IV) with a good performance status is represented by chemotherapy with platinum derivatives cisplatin or carboplatin in combination with gemcitabine, paclitaxel or nab-paclitaxel and, eventually, radiotherapy for palliation (4-6). In this light, patients with advanced sqNSCLC have a poor prognosis with a survival that usually does not exceed 9–10 months and with no real improvement attained with systemic treatments in the last three decades (4). The long-lasting therapeutic failure for advanced sqNSCLC patients has been recently interrupted by the positive results of clinical trials testing programmed-cell-death-receptor-1 (PD-1)/programmed-cell-death-receptor ligand-1 (PDL-1) immune-checkpoint blockade with monoclonal antibodies (mAbs) such as Nivolumab, Pembrolizumab or Atezolizumab. These results lead the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) to approve some of these agents for the second line treatment of advanced sqNSCLC patients (7-10). We have previously designed a multistep phase I/II clinical trial (Beva 2007 study) aimed to investigate the toxicity, the biological and anti-tumor activity of a novel metronomic chemotherapy regimen with dose-fractioned cisplatin and oral etoposide (mPE) +/− bevacizumab (mPEBev) in NSCLC patients, including the squamous cell histology. Metronomic chemotherapy is an emerging treatment modality for cancer patients based on the use of cytotoxic drugs administered at lower dosage for a prolonged period of time (11). This modality allows to achieve a higher dose intensity of cytotoxic drugs compared to traditional modality avoiding dangerous spikes in blood concentration (12,13). Some of these properties may be enforced by a rationale combination with bevacizumab, a humanized IgG1 to the vascular endothelial growth factor (VEGF), able to increase the efficacy of standard poly-chemotherapy in NSCLC patients (14,15). Metronomic chemotherapy and VEGF deprivation by bevacizumab synergize as both anti-angiogenic and immune-modulating activity, resulting in a significant antitumor activity. Even though considered a very active drug for NSCLC patients, bevacizumab was not approved for the treatment of sqNSCLC due to the high risk of bleeding reported in the early trials. This risk was correlated to the central localization of the disease that often infiltrates the large mediastinum vessels. Considering the lowest dosage and chronic administration of the treatment, our trial also included NSCLC patients with squamous histology with low risk of bleeding. The mPEBev regimen resulted safe and very active with a partial response and disease stabilization rate of 68.8% and 17.8%, respectively, and a progression free survival (PFS) of 9.5 months (16,17). The treatment was associated to a fast and progressive decline in the tumor blood flux (perfusional CT scan) paralleled by a progressive decline in the serum levels of pro-angiogenic and immune-modulating cytokines (VEGF, angiopoietin-1, thrombospondin-1, follistatin, IFNɣ, IL-4 and IL-17A), and inflammatory markers [neutrophil-lymphocyte ratio (NLR), C reactive protein (CRP), lactate dehydrogenase (LDH), myeloperoxidase] (17,18). Additional in vivo and ex vivo immunological studies also revealed a treatment-related improvement in both tumor antigen processing and presentation ability by active peripheral dendritic cells (DCs) and a more efficient tumor-specific cytotoxic T cell (CTL) response (18) suggesting that either mPE or mPEBev regimens may improve the micro-environmental conditions necessary for an efficient anti-tumor activity by antigen specific T cell effectors. At this purpose, we performed a retrospective analysis aimed to evaluate the antitumor activity of the mPE regimen +/− bevacizumab, in the subset of patients with sqNSCLC histology enrolled in the second step of the BEVA2007 trial and, also, carried out a statistical analysis aimed to identify possible immunobiological markers predictive of positive outcome in these patients.
Methods
Study design
The study protocol code #BEVA2007 (2008-006051-40) was a two-step phase I/II clinical trial, performed in accordance to the good clinical practice guidelines and was approved by the Bioethics Committee of the University of Siena as described in previous reports (16-18). The first step of the study included 25 patients, who were sub-divided in five cohorts receiving escalating dosage of bevacizumab. Cohort 1 received mPE chemotherapy alone, while cohort 2, 3, 4 and 5 received bevacizumab every three weeks, at the dosage of 2.5, 5, 7.5 and 10 mg/kg every three weeks. This first step revealed coincidence of bevacizumab maximal tolerated dose (MTD) and most effective biological dose (MEBD) at 5 mg/kg (16), which was chosen as standard dosage for the second step of the study. The inclusion criteria were: histological diagnosis of mNSCLC, performance status (ECOG) from 0 to 2, normal renal and hepatic function, WBC count more than 2,500/mm3, hemoglobin more than 9 g/dL, platelet cell count more than 90,000/mm3, normal cardiac function. The exclusion criteria were: Central tumors with high risk of bleeding (excavated with large necrosis and infiltration of large arterial and venous structures) for bevacizumab use, a history of other severe cardiovascular disease, arrhythmia, second malignant tumors, signs of active infections. The trial included a calibration group of thirty patients who were aimed to receive the same metronomic chemotherapy and no bevacizumab. Four patients in the mPEBev group and two patients in the calibration group retired the consent and did not receive the treatment.
Treatment schedule
Eighty six patients received, every three weeks, iv. cisplatin (30 mg/sqm) on days 1–3 and daily oral etoposide (50 mg) on days 1–15 and bevacizumab, 5 mg/kg, on the day 3 for a maximum of four consecutive courses (16-18). In the calibration group, twenty eight patients received the same metronomic chemotherapy with no bevacizumab administration. The response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Biological analysis and blood sampling
Peripheral blood samples (10 mL) were withdrawn at baseline and one hour before any treatment cycle for both serum and PBMC isolation. Serum derived from standard peripheral blood centrifugation and peripheral blood mononuclear cells (PBMCs), obtained by Ficoll-Hypaque (Celbio S.P.A., Italy) gradient separation medium from heparinized blood samples, were immediately frozen and stored as described in previous studies (16-18). Lymphocytes, platelets, neutrophils and monocytes were evaluated by hemocytometric cell counts, while their feature was evaluated by microscope analysis. Flow cytometry was performed on patients’ PBMCs by carrying out standard multicolor immuno-cytoflurimetric analysis with conjugated anti-CD3, CD4, CD8, CD27, CD62L, CD19, CD16, CD56, CD25, FoxP3, CCR7, CD45Ra, CD11b, CD11c, CD14, CD15, all purchased by Bioscience, USA.
BioPlex assay
Blood samples were collected from a peripheral vein at baseline and after 3 treatment courses and kept on ice. Serum was collected by centrifugation (3,000 rpm for 10 min at 4 °C), aliquoted and stored at −80 °C until analyzed. A multiplex biometric ELISA-based immunoassay, containing dyed microspheres conjugated with a monoclonal antibody specific for a target protein was used according to the manufacturer’s instructions (Bio-Plex, Bio-Rad Lab., Inc., Hercules, CA, USA). Soluble molecules were measured using either commercially available kits or customized kits for the evaluation of the following cytokines: interleukin (IL)-4, IL-8, IL-10, IL-12, IL-17, interferon (IFN)ɣ, tumor necrosis factor (TNF)α, VEGF, Granulocyte-Colony Stimulating Factor (GCSF) and angiopoietin-2, as described in previous papers (16). Serum levels of all proteins were determined using a Bio-Plex array reader (Luminex, Austin, TX, USA) that quantifies multiplex immunoassays in a 96-well plate with very small fluid volumes. The analyte concentration was calculated using a standard curve, with software provided by the manufacturer (Bio-Plex Manager Software).
Statistical analysis
The between-mean differences were statistically analyzed using Stat View statistical software (Abacus Concepts, Berkeley, CA, USA). The results were expressed as the mean +/− standard deviation (SD) of four determinations made in three different experiments, and the differences determined using the 2-tail Student’s t-test for paired samples. In order to perform a survival analysis we divided the patients into two subgroups with low (L) and high (H) score, according to their respective median value of each specific marker or treatment related level change expressed as fold change to baseline value. Kaplan Meier’s method and Log-Rank test were used to evaluate PFS and OS and correlate them with patients’ associated variables. All analyses were performed by using SPSS statistical package, version 17.0. A P value of 0.05 or less was considered statistically significant.
Results
The BEVA2007 study was a multistep phase I–II trial aimed to investigate, in advanced NSCLC patients, the safety, immunobiological and antitumor activity of the mPE doublet +/− bevacizumab, an original metronomic chemo-biological regimen that showed significant anti-angiogenic and immunological activity in previous studies (13,16-18). One hundred twenty advanced NSCLC patients were enrolled in the study between September 2007 and September 2015 and one-hundred sixteen of them received the treatment. Of the 116 patients receiving the treatment, 62 patients presented a histological diagnosis of adenocarcinoma, 31 of squamous cell carcinoma and the remaining 23 of other NSCLC subtypes. We carried out our retrospective study in the squamous cell subset; in this group, there were 29 males and 2 females, with median age of 67 years and median performance status of 1, according to the ECOG score. At the time of diagnosis, 6 out of 31 of these patients resulted in a stage IIIB and 25 out of 31 in a stage IV. Seventeen out of 31 patients received frontline chemotherapy according to the mPE regimen, while 14 out of 31 received the mPE chemotherapy in combination with bevacizumab.
Clinical results
The treatment was safe in patients with advanced sqNSCLC. They received a median number of four treatment courses with no significant toxicity or toxicity-related delay among the chemotherapy courses. No toxic death, bleeding episodes or severe infections were recorded; however, we recorded slight hematological toxicity mainly consisting in reversible grade 1–3 leukopenia (6 cases) rapidly recovered with the use of growth factors, grade 2 anaemia (6 cases), grade 1–2 gastroenteric toxicity (3 cases), grade 2–3 infections (3 cases) and alopecia (16 cases). Two patients required blood transfusions after three treatment courses and required a 25% cisplatin dose reduction. The latter two patients did not show any sign of bleeding and the anaemic state was consistent with a clinical picture of cisplatin related haematological toxicity which also involved blood cells and platelets. No case of treatment-related lethargic encephalitis or lung fibrosis was recorded in these patients; this was not surprising considering that bevacizumab was administered at the dosage of 5 mg/kg which is two/three times lower than that commonly used for the treatment of non-squamous NSCLC patients. The treatment showed a promising antitumor activity in these sqNSCLC patients; in fact, complete response (histologically confirmed) was obtained in 1, a partial response in 17 out of 31, a stable disease in 4 out of 31 and a progressive disease in the remaining 9 out of 31 patients, respectively. We also recorded a mean PFS and OS of 13.6±4.7 [95% confidence interval (CI): 4.344–22.888] and 17±4.4 (95% CI: 8.372–25.736) months (Figure 1A,B), respectively. Six out of 8 patients who showed a noteworthy tumor shrinking were subjected to lung surgery for tumor resection after four treatment courses. These patients gained a significant advantage in term of survival [36.6±15.95 (5.3–67.8) vs. 11.4±1.47 (8.54–14.3) months; P=0.048] (Figure 2A,B,C).
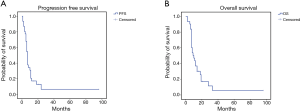
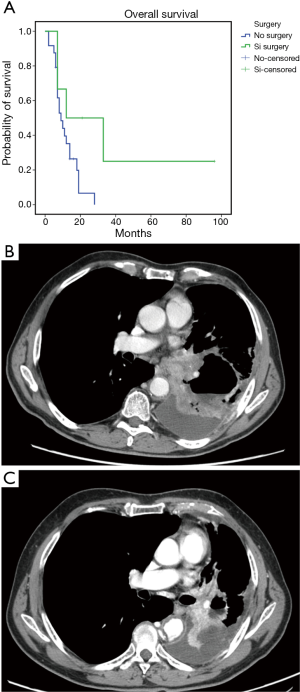
Biological correlations
The mPE chemotherapy regimen +/− bevacizumab showed anti-angiogenic and immunological effects also for patients with squamous cell histology. In fact, in this patients’ subset, it was observed a treatment-related decline in serum levels of VEGF and IL-17, paralleled by a significant increase in peripheral blood of activated CTLs (CD8+CD62L+), central memory T cells (Tcms) (CD8+CD45Ra−CCR7+) and cell lineages expressing the phenotype of activated DCs expressing CD83 and the dominant co-accessory molecule CD80. In this patients’ subset our analysis failed to demonstrate statistically significant differences related to the presence or absence of bevacizumab in the treatment. Therefore, we investigated whether these immunological events, measured at baseline and after three treatment courses, correlated with patients’ outcome by performing Kaplan Meyer curves and log-rank tests. Our analysis recorded a much longer survival in those patients who presented lower baseline levels of neutrophil-to-lymphocyte ratio (NLR) [L vs. H: 24.95±8.7 (7.80–42.04) vs. 9.88±3.77 (2.49–17.27) months, P=0.05], IL-17A [L vs. H: 4.63±13.6 (7.84–61.44) vs. 11.0±1.1 (5.14–16.85) months, P=0.036] (Figure 3 A,B), peripheral regulatory-T-cells (Tregs) [L vs. H: 40.14±16.6 (7.57–72.7) vs. 9.9±2.04 (5.9–13.9) months, P=0.02] and higher peripheral levels of activated CD83+DC [L vs. H: 8.4±2.01 (4.45–12.34) vs. 24.0±6.06 (12.12–35.88) months, P=0.03] (Figure 4A,B). These data support the hypothesis of a strong involvement of immune-system in the outcome of these patients. Patients who presented a treatment-related decrease in IL-17A levels showed a trend to a longer survival, which did not achieve statistical significance, probably due to the small statistical patient sample. No significant differences were, on the other hand, observed for other examined parameters.
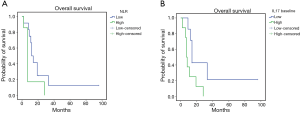
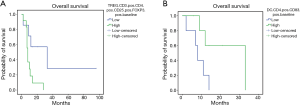
Discussion
Our retrospective analysis, carried out on a subset of thirty-one advanced sqNSCLC patients enrolled in the second step of BEVA2007 trial, revealed a 71% disease control rate (complete response, partial response and stable disease) with a PFS and OS respectively of 13.6±4.7 (95% CI: 4.34–22.88) and 17±4.4 (95% CI: 8.372–25.736) months and a 42% one-year survival rate. Additionally, 6 (19.3%) patients were down-staged after this treatment and could subsequently receive radical surgery achieving a median survival of 36 months. Even though significant bias does exist, due to both the small statistical sample of the patients and the retrospective nature of our analysis, these results appear promising considering that the most commonly used chemotherapy doublets induce in these patients a response rate of 25–40% and a median survival no longer than 9–10 months (4,6). In particular, we found that a systemic baseline inflammatory profile characterized by a low LNR, low levels of IL-17A and Tregs, and a high baseline expression of active DCs (CD83+), is predictive of longer survival (19-21). It has been proposed that a chronic inflammation status supports tumor progression by increasing growth factors, chemokines and cytokines; they also promote inflammation-related neo-angiogenesis and both homing and differentiation of immune-suppressive cell lineages like myeloid derived suppressive cells (MDSCs) and Tregs (21-25). Tregs, whose activation is under control of CTLA-1 checkpoint, have the specific ability to attenuate the extent of cancer associate immune-response and represents a common mechanism of immune-escape for cancer cells (26-29). IL-17A is able to promote the switch of inactive Tregs in highly suppressive subsets that, in turn, can inhibit all the attempts of immune-system and tumor-specific CTLs to counteract tumor growth and development (29-32). The decreased levels of VEGF and IL-17A following mPE/mPEBev regimen were paralleled by the increased percentage of peripheral Tcms and activated CD62L+CTLs and by the expansion of activated myeloid derivative DCs expressing CD83 and CD80. Tcms down-regulate CCR-7 expression and differentiate in highly cytotoxic effector cells (effector memory (Tem)/CD8+CD45Ra-CCR7-) or long term memory T cells (CD27+) (33-36). An increase of Tcms, therefore, represents a new source of antigen specific effector cells able to sustain a prolonged immunization with tumor-specific cytolytic activity (33-36). In this light, CD8+CD62L+, is another very active CTL subset expressing the L selectin (CD62L), a trans-membrane protein which allows the binding to the specific receptor on tumor vessels and the consequent extravasation in the tumor sites (37). In our patients, we also found a significant increase of peripheral DCs expressing CD83 and CD80, a very efficient antigen presenting cell linage able to uptake and process antigen released by tumor tissues exposed to the cytotoxic drugs (38). Moreover, we recorded a longer survival in those patients who showed a higher treatment-related increase in activated DCs. These immunological effects, also described in previous studies (13,16-18), have been partially related to the metronomic modality of administration and can be also sustained by bevacizumab. The latter is known for its ability in inhibiting endothelial cell proliferation and neo-vessel formation and in inducing vessel normalization in cancer patients; however, it also promotes neutrophils’, MSDCs’, and Tregs’ maturation and induces inhibitory effects on DC maturation and CTL precursors’ activation (39-42). On this basis, it is not surprising that our metronomic chemotherapy +/− bevacizumab exerts immunological effects that may affect NSCLC patients’ survival. These results may acquire additional interest for the recent development of immunotherapy based on PD-1/PDL1 immune-check point inhibitors in the treatment of mNSCLC, including squamous cell carcinoma (43-48). In this view, the presence of PD1+ T cells in tumor tissue is a consequence of pre-existing tumor specific immune-response that can be restored by anti-PD-1/PDL-1 mAbs such as nivolumab, pembrolizumab, and atezolizumab. These agents have shown promising antitumor activity in different neoplasms including melanoma, NSCLC, kidney and colon cancer and it is clear that their efficacy is strictly related to a pre-existing antitumor immune-response, which has been at least partially attenuated by PD-1/PDL-1 checkpoint and that is the ultimate weaponry able to kill tumor cells.
Conclusions
Based on our results, we believe that a possible way for improving the efficacy of mAbs in sqNSCLC patients could consist in testing a frontline treatment like mPEBev that mobilizes a large number of immune cells inducing an antitumor immunization followed by PD-1/PDL-1 checkpoint inhibitors. In conclusion, the mPE +/− bevacizumab regimen is an active treatment for advanced sqNSCLC patients and deserves additional evaluation in larger studies. Moreover, the immunological effects recorded in this trial can represent a solid basis to propose the mPE +/− bevacizumab regimen in a sequential combination with PD-1/PDL-1 immune-checkpoint inhibitors.
Acknowledgements
This work was supported by the Italian Ministry of University and Research (FIRB-ACCORDI DI PROGRAMMA 2011 and Regione Campania, Laboratori Pubblici Hauteville).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol code #BEVA2007 (2008-006051-40) was a two-step phase I/II clinical trial, performed in accordance to the good clinical practice guidelines and was approved by the Bioethics Committee of the University of Siena. One-hundred twenty patients signed a written informed consent and were enrolled in the second step of the study.
References
- GLOBOCAN 2012: Estimated Cancer incidence, Mortality and Prevalence Worldwide in 1012. World Health Organization, International Agency for research on cancer. [accessed 2016 May 10]. Available online: http://globocan.iarc.fr/Default.aspx
- Ettinger DS. Lung Cancer and other pulmonary neoplasms. In: Goldman L, Schafer AI. editors. Goldman's Cecil Medicine. 24th ed. New York: Elsevier, 2012:1264-7.
- Pass HI, Carbone DP, Johnson DH, et al. editors. Principles and Practice of Lung Cancer: the official reference text of the International Association for the Study of Lung Cancer (IASLC). Philadelphia: Lippincott Williams & Wilkins, 2012.
- Pilkington G, Boland A, Brown T, et al. A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax 2015;70:359-67. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small cell-lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [Crossref] [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first line treatment in stage IIIB/IV non-small-cell-lung cancer: results from a randomized double blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab vs docetaxel in advanced squamous cell non-small-cell-lung cancer. New Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrulizumabvsdocetaxel for previously treated PDL-1 positive advanced non-small-cell-lung cancer (Key note 010): A randomized controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezulimumab vs docetaxel for patients with previously treated non small cell lung cancer (POPLAR): A multicenter, open label phase II randomized controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Maiti R. Metronomic chemotherapy. J Pharmacol Pharmacother 2014;5:186-92. [Crossref] [PubMed]
- Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett 2015;358:100-6. [Crossref] [PubMed]
- Correale P, Cerretani D, Remondo C, et al. A novel metronomic chemotherapy regimen of weekly platinum and daily oral etoposide in high-risk non-small cell lung cancer patients. Oncol Rep 2006;16:133-40. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non small cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first line therapy of non squamous non small cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Correale P, Remondo C, Carbone FS, et al. Dose/dense metronomic chemotherapy with fractioned cisplatin and oral daily etoposide enhances the anti-angiogenic and anti-tumor activity of bevacizumab in advanced non-small-cell-lung cancer patients. Cancer Biol Ther 2010;9:685-93. [Crossref] [PubMed]
- Correale P, Botta C, Basile A, et al. Phase II trial of bevacizumab and dose/dense chemotherapy with cisplatin and metronomic daily oral etoposide in advanced non-small-cell-lung cancer patients. Cancer Biol Ther 2011;12:112-8. [Crossref] [PubMed]
- Martino EC, Misso G, Pastina P, et al. Immune-modulating effects of bevacizumab in metastatic non-small-cell-lung cancer patients. Cell Death Discov 2016;2:16025. [Crossref] [PubMed]
- Botta C, Barbieri V, Ciliberto D, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther 2013;14:469-75. [Crossref] [PubMed]
- Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013;13:158. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 2012;23 Suppl 8:viii6-9.
- Jensen-Jarolim E, Singer J. Cancer vaccines inducing antibody production: more pros than cons. Expert Rev Vaccines 2011;10:1281-9. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Predina J, Eruslanov E, Judy B, et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc Natl Acad Sci USA 2013;110:E415-E424. [Crossref] [PubMed]
- Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151-64. [PubMed]
- Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell 2008;133:775-87. [Crossref] [PubMed]
- Brzostek J, Gascoigne NR, Rybakin V. Cell Type-specific regulation of immunological synapse dynamics by B7 ligand recognition. Front Immunol 2016;7:24. [Crossref] [PubMed]
- Lu LF, Rudensky A. Molecular orchestration of differentiation and function of regulatory T cells. Genes Dev 2009;23:1270-82. [Crossref] [PubMed]
- Pan B, Shen J, Cao J, et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep 2015;5:16053. [Crossref] [PubMed]
- Duan MC, Zhong XN, Liu GN, et al. The Treg/Th17 paradigm in lung cancer. J Immunol Res 2014;2014:730380. [Crossref] [PubMed]
- Zhang GQ, Han F, Fag XZ, et al. CD4+, IL17 and FoxP3 epression in different pTNM stages of operabe Non-small-cell lung cancer and effects on disease prognosis. Asian Pac J Cancer Prev 2012;13:3955-60. [Crossref] [PubMed]
- Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708-12. [Crossref] [PubMed]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004;22:745-63. [Crossref] [PubMed]
- Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol 2005;42:799-809. [Crossref] [PubMed]
- Höpken UE, Winter S, Achtman AH, et al. CCR7 regulates lymphocyte egress and recirculation through body cavities. J Leukoc Biol 2010;87:671-82. [Crossref] [PubMed]
- Jackson SS, Schmitz JE, Kuroda MJ, et al. Evaluation of CD62L expression as a marker for vaccine–elicited memory cytotoxic T lymphocytes. Immunology 2005;116:443-53. [PubMed]
- Martin K, Schreiner J, Zippelius A. Modulation of APC function and anti-tumor immunity by anticancer drugs. Front Immunol 2015;6:501. [Crossref] [PubMed]
- Alfaro C, Suarez N, Gonzalez A, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer 2009;100:1111-9. [Crossref] [PubMed]
- Terme M, Colussi O, Marcheteau E, et al. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol 2012;2012:492920. [Crossref] [PubMed]
- Correale P, Cusi MG, Tagliaferri P. Immunomodulatory properties of anticancer monoclonal antibodies: is the ‘magic bullet’ still a reliable paradigm? Immunotherapy 2011;3:1-4. [Crossref] [PubMed]
- Voron T, Marcheteau E, Pernot S, et al. Control of the Immune Response by Pro-Angiogenic Factors. Front Oncol 2014;4:70. [Crossref] [PubMed]
- Boussiotis VA, Chatterjee P. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J 2014;20:265-71. [Crossref] [PubMed]
- Luke JJ, Ott PA. PD-1 pathway inhibitors: The next generation of immunotherapy for advanced melanoma. Oncotarget 2015;6:3479-92. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune check points in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control 2014;21:80-9. [Crossref] [PubMed]
- Brahmer JR, Hammers H, Lipson EJ. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future Oncol 2015;11:1307-26. [Crossref] [PubMed]
- Sundar R, Cho BC, Brahmer JR, et al. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 2015;7:85-96. [Crossref] [PubMed]


