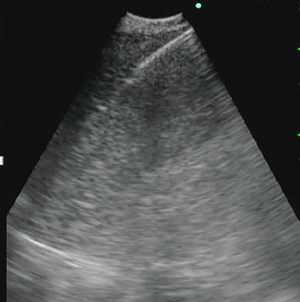
Comparison of the yield of 19-G eXcelon core needle to a 21-G EBUS needle during endobronchial ultrasound guided transbronchial needle aspiration of mediastinal lymph nodes for the detection of granulomas in cases of suspected sarcoidosis
Granulomatous diseases are thought to be the most common cause of mediastinal adenopathy (1). Endobronchial ultrasound aided bronchoscopic transbronchial needle aspiration (EBUS-TBNA) provides us with a mechanism to perform lymph node biopsies under real time ultrasound guidance (1). However, the diagnostic performance of EBUS-TBNA using the traditional 21-G needle is obfuscated to some extent because of the lack of its ability to supply core biopsy from lymph nodes and hence failing to demonstrate granulomas in a significant number of cases (15%) (2). The diagnosis of sarcoidosis requires the clear demonstration of a non-caseating granuloma (3). Some authors have used the presence of epithelioid cells alone from EBUS-TBNA samples for diagnosing sarcoidosis which has resulted in >90% sensitivity for the procedure using the 21-G EBUS needle but further analysis based on presence of granulomas have reduced their yields to <70% (4). The 19-G core needle used for conventional TBNA (c-TBNA) can provide a histological core (5). Although achieving histological cores with a 22-G needle has been previously reported (in 92% cases), the relevance of such “core biopsies” is unclear (6). Cores were reported to be available in 92% of 22-G EBUS-TBNA samples. However, this report goes on to document that histological material leading to diagnosis was available in only 57% of the cases (6). It leads us to question whether such histological cores obtained by the 22-G needle are of the same diagnostic utility as a true core biopsy. Thus, one well-recognized drawback of EBUS-TBNA has been lack of preserved cellular architecture on needle aspirates using the 22-G and 21-G needles. Few studies have addressed the questions whether a 21-G EBUS-TBNA needle has a higher diagnostic yield compared to a 22-G needle. Unfortunately, the verdict is far from being clear (7). Hence, we have used the 19-G conventional TBNA needle to perform a hybrid EBUS TBNA for those patients with an initial negative 21-G EBUS-TBNA biopsy yet have a high possibility of having sarcoidosis. We aim to review our single center experience with the 19-G core needle biopsy used with an EBUS bronchoscope in this paper. This is a hybrid procedure that used a 19-G c-TBNA needle fitted to a regular EBUS bronchoscope in order to provide core samples under direct visualization. The procedure is described in the online supplement. We then systemically evaluated whether performing EBUS with a 19-G core needle resulted in an increased yield of granulomas than regular EBUS TBNA with a 21-G needle alone. A specific 19-G EBUS-TBNA needle was not commercially available at the time these procedures were performed and hence, we preferred to use this hybrid method for only those patients who had an inconclusive initial 21-G EBUS-TBNA evaluation rather than all patients requiring a biopsy. All study specimens were retrieved and retrospectively evaluated by a single pathologist. We applied the criteria for diagnosis as suggested by the American Thoracic Society (ATS)/European Respiratory Society (ERS)/World Association of Sarcoidosis and Other Granulomatous Disease (WASOG) statement on sarcoidosis. It emphasizes that the histologic diagnosis of sarcoidosis depends on the demonstration of tight, well-formed granulomas and a rim of lymphocytes and fibroblasts in the outer margin of granulomas (3).
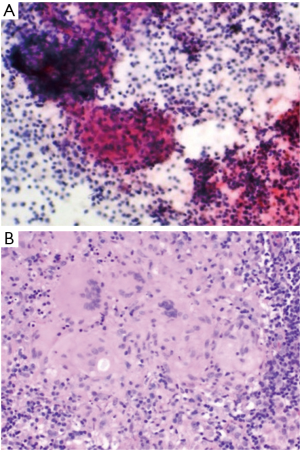
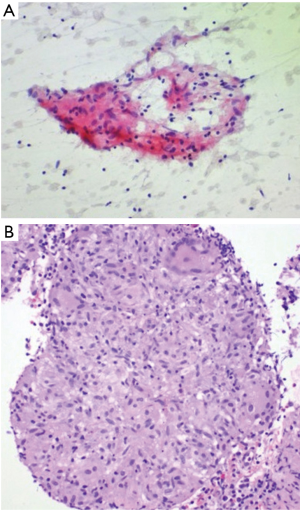
Six men and five women, with an initial negative 21-G EBUS-TBNA biopsy procedure, underwent repeat EBUS bronchoscopy (Table 1) for suspected sarcoidosis using a 21-G needle as well as 19-G core needle biopsy during this study period. Ten subcarinal lymph nodes and one right paratracheal lymph node were examined. One lymph node per patient was examined. Of the 11 patients with suspected sarcoidosis, sarcoidosis was diagnosed in ten patients. One patient had benign non-sarcoid granuloma (GLUS). All eleven patients reviewed had well-formed visible granulomas on histological evaluation from the 19-G needle. Among the 21-G needle fine needle aspiration (FNA) cytology specimens, a non-caseating granuloma was visible in two patients. Reactive inflammation was present in two other patients. All eleven patients had better preservation of cellular architecture on samples obtained using the 19-G needle (Figures 1,2). There were no procedural complications in any of the patients undergoing the hybrid procedure. There was no damage to the bronchoscope. This study demonstrates the advantages of using a bigger 19-G core needle which we feel, has provided greater tissue and subsequently allowed us to make a definitive diagnosis of sarcoidosis in those cases that have failed an initial 21-G EBUS-TBNA evaluation. The material (aggregates of histiocytes) obtained from the 21-G needle were often so small that it is sometimes difficult to comment if they are normal lymph node histiocytes or an actual granuloma. However, with the 19-G core needle, a better view of the tight, palisaded architecture helped the pathologist identify a granuloma with greater degree of confidence. We would expect better yields with the utilization of the newer 19-G EBUS-TBNA needle when applied to the same clinical question. The use of an EBUS-Core biopsy may also be helpful when the diagnosis of sarcoidosis is clinically unclear and other granulomatous conditions are being considered.

Full table
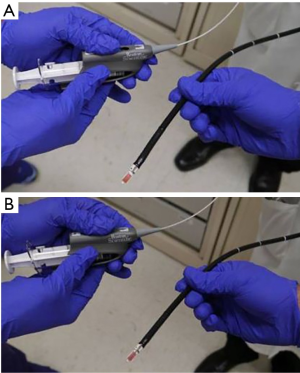
A real-time EBUS-TBNA scope (BC-UC180F; Olympus Medical Systems Corp, Tokyo, Japan, 7.5 MHz) was used in all cases. After locating the target lymph node using ultrasound guidance a 21-G needle (Olympus 21-G Vizishot, NA-201SX-4021) was used to obtain a transbronchial needle aspiration. Three to five passes were made under direct ultrasound visualization. Direct-smear technique was used for preparation of the 21-G TBNA samples. Needle content was coated on a glass slide, fixed with 95% alcohol, and then stained using a standard Papanicolaou stain. The remnants of aspirates were collected in Roswell Park Memorial Institute (RPMI) media fluid by rinsing the biopsy needle. This was processed into cell block material and stained with a standard H & E stain. On site cytology evaluation was used to ensure adequacy of the specimen. Once EBUS-TBNA using the 21-G needle was completed, a 19-G needle (Boston Scientific eXcelon) was inserted into the bronchoscope and samples were obtained as described in annexe. A core sample was first collected and placed in 10% formalin. Next, using the same technique as for the 21-G needle, glass slides were prepared immediately fixed with alcohol (95%) for cytological evaluation. Material for cell block material was collected using the same process as for the 21-G needle in RPMI media. All smears were checked for acid fast bacilli (AFB) and fungus using gomori methenamine-silver stains. All specimens were sent to pathology for histopathological (19-G) and cytological (21-G) evaluation.
Hybrid method: the conventional 19-G TBNA needle was introduced into the working channel of a convex probe EBUS long enough to have its needle tip visible at the end of the probe (Figure S1). The position at the shaft of the needle at the working channel was marked (Figure S2). These two steps are performed before introducing the scope into the patient’s airway. The EBUS scope was introduced through the oropharynx and trachea in the usual fashion and the lymph node was visualized. Then the needle was introduced up to the previously determined length (limited to the previously made mark). The needle tip would be seen on the convex probe ultrasound screen. The assistant would stabilize the bronchoscope in place and the operator would briskly push the needle shaft into the lymph node based on the size of lymph node (Figures S3,S4). This would usually vary between 0.5 to 1.5 cm. The needle tip would be seen to pass through the airway wall and enter the lymph node itself (Figure S5). It would be agitated slightly within the node to allow lymph node tissue to enter the hollow needle. The needle would be taken out and samples prepared each time before returning it back to its position taking care not to push it past the predetermined mark. Three such passes would be done with the needle.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Olak J. Benign lymph node disease involving the mediastinum. In: Shields TW, LoCicero J, Reed CE, et al. editors. General Thoracic Surgery. 7th edition. Philadelphia: Lippincott Williams & Wilkins, 2009:2366-7.
- Navani N, Booth HL, Kocjan G, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology 2011;16:467-72. [Crossref] [PubMed]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-55. [PubMed]
- Nakajima T, Yasufuku K, Kurosu K, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis–comparisons with other bronchoscopic diagnostic modalities. Respir Med 2009;103:1796-800. [Crossref] [PubMed]
- Cağlayan B, Salepçi B, Fidan A, et al. Sensitivity of convex probe endobronchial sonographically guided transbronchial needle aspiration in the diagnosis of granulomatous mediastinal lymphadenitis. J Ultrasound Med 2011;30:1683-9. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology 2007;12:863-8. [Crossref] [PubMed]
- Muthu V, Gupta N, Dhooria S, et al. A prospective, randomized, double-blind trial comparing the diagnostic yield of 21- and 22-gauge aspiration needles for performing endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis. Chest 2016;149:1111-3. [Crossref] [PubMed]