AB thymoma with atypical type A component with delayed multiple lung and brain metastases
Introduction
Thymomas are uncommon tumors originating from the epithelial cells of the thymus. In 1999 the World Health Organization (WHO) committee proposed a histological classification system for thymic epithelial tumors, distinguishing five main histological types of thymomas: A, AB, B1, B2 and B3. This is based on the shape of the neoplastic cells and the amount of the lymphocytic component (1). A, AB and B1 thymomas are regarded as being less aggressive types, while B2 and B3 thymomas behave more aggressively. The revised WHO classification of 2004 included the genetic alterations correlating with the histological subtypes of thymomas and clinical behavior (2). In 2015, the most recent WHO classification maintained the previous subdivision but included an atypical variant of type A thymoma (3,4). This variant was first described by Nonaka et al. (5) and is characterized by hypercellularity, increased mitotic activity and necrosis. The very recent observation of rare atypical type AB thymoma with increased mitotic count, necrosis and nuclear pleomorphism was also mentioned but due to the paucity of such cases (6,7), a subtype extra-chapter about “atypical type AB thymoma” was not introduced into the classification. Since all major histological thymoma subtypes can metastasize, they are considered as malignant. However, they are usually only locally aggressive tumors and metastases to distant sites are infrequent, occurring in less than 3% of cases across all histological types. Lung, regional nodes and liver are the commonest metastatic sites, while their spreading to the central nervous system (CNS) is extremely rare.
Case presentation
Primary mediastinal tumor
A 66-year-old woman was admitted to the Clinics of Surgery in 2000 due to a mediastinal tumor that was accidentally detected on chest X-rays 3 months prior to admission. The patient underwent sternotomy with resection of the tumor and mediastinal fat tissue.
The resected tumor measured 13 cm × 8.5 cm × 6 cm. Macroscopically, it was encapsulated and firm with areas of necrosis. Microscopic examination revealed that majority of the neoplasm was composed of areas rich in Tdt-positive lymphocytes intermingled with spindle epithelial cells (Figure 1) positive for cytokeratin (AE1AE3), p40 and CD20. This component was originally diagnosed as a “mixed thymoma”.
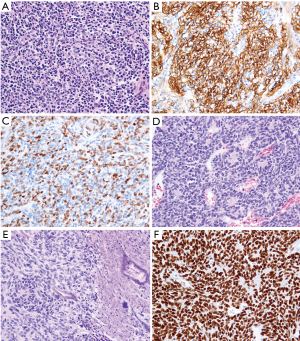
There were also lymphocyte-poor lobules that constituted up to 10% of the tumor with the architecture sharing the features of hemangiopericytoma and carcinoid. Immunohistochemical analysis with anti-chromogranin A and synaptophysin antibodies excluded neuroendocrine differentiation. The neoplastic cells revealed expression of cytokeratin (AE1AE3), p40 and PAX-8 and were negative for CD5, CD117 and CD20. The epithelial cells were ovoid with small nucleoli, densely packed often forming small nests, trabeculas or rosettes. The mitotic index reached 12 mitotic figures per 10 HPF (high power fields). The tumor revealed foci of necrosis. Numerous thin-walled, slit-like or dilated vessels were dispersed throughout the tumor, but no perivascular spaces were observed. Only single Tdt-positive lymphocytes were found. These areas of the tumor were originally named “well-differentiated thymic carcinoma” to emphasize their atypical appearance, although the morphologic features did not fulfill adequate criteria of this entity.
Based on above findings, the original diagnosis was “mixed thymoma with a well-differentiated thymic carcinoma component” following the nomenclature of the Marino and Müller-Hermelink classification (8) modified by Kirchner et al. (9) The neoplasm traversed the capsule and invaded perithymic fat tissue, thus fulfilling the criteria of a Masaoka-Koga stage II thymoma (6). Surgical margin was free of neoplastic tissue. Due to the tumor stage, the patient received adjuvant radiotherapy.
According to the criteria of the current WHO classification [2015], the above primary thymic tumor should be diagnosed as “AB thymoma” instead of “mixed thymoma”. Furthermore, the minor lymphocyte-free areas with necrosis and increased mitotic activity that were originally diagnosed, as “well-differentiated thymic carcinoma“ would currently be described as type A areas with atypia in an AB thymoma. Thus, the current diagnosis of the primary tumor ought to be AB thymoma with atypical type A areas, i.e., “atypical type AB thymoma” (7).
Lung metastases
Ten years after the surgery five nodules, measuring 0.5–2 cm in diameter, were detected on routine follow-up CT in the lower right lung lobe. The metastases of the thymoma were taken into consideration. The nodules were metabolically active on Positron Emission Tomography (PET) examination and removed by lobectomy.
Microscopically, all metastatic tumors corresponded morphologically to the carcinoid-like/hemangiopericytoma-like component observed in the primary thymoma (Figure 2). No foci of necrosis were found. Neoplastic cells revealed diffuse immunoreactivity with AE1AE3, p40 and PAX-8 and patchy reaction with CD20 antibody. They were negative for neuroendocrine markers, CD5 and CD117. Their mitotic index reached 16 mitotic figures/10 HPF. A certain number of Tdt-positive lymphocytes was scattered focally among epithelial cells. Since no lymphocyte-rich areas were detected, the diagnosis according to the new WHO classification would be “atypical type A thymoma metastatic to the lung”.

The patient did not receive any adjuvant treatment after excision of the metastases.
Brain metastasis
In 2015, the patient was admitted to the Department of Neurosurgery due to a mild transcortical motor dysphasia and a discrete right hemiparesis. On admission, the patient presented minimal symptoms of increased intracranial pressure. Magnetic resonance imaging (MRI) of the brain revealed a large frontal lobe tumor mass with a significant cystic component (Figure 3). A left frontal craniotomy with gross total tumor resection was performed. The initial postoperative course was uneventful, however, 15 days after neurosurgery the patient developed bilateral pneumonia and, subsequently, status epilepticus and died 25 days later despite intensive treatment.

Microscopically, the brain tumor revealed the predominance of oval or spindle-shaped epithelial cells arranged in solid sheets or in a more conspicuous storiform or fascicular pattern. The majority of the neoplastic tissue showed prominent hemangiopericytoma-like growth pattern with numerous branching vascular staghorn-like spaces (Figure 4). Foci of necrosis were found. Mitotic activity was brisk (about 15 mitotic figures/10 HPFs). Lymphocytes were absent or only scattered within the spindle cell component a few of them revealed Tdt expression. The tumor cells demonstrated strong diffuse immunopositivity for pancytokeratin, CK8, and CK18, patchy positive reaction with CD20 and negative reaction for CD34 and STAT6. The tumor cells exhibited strong nuclear expression of p40. Ki67 proliferative index was established on about 25%.
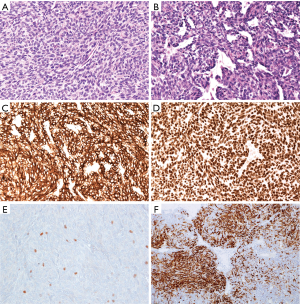
The diagnosis of a solitary fibrous tumor/hemangiopericytoma was taken originally into consideration, however, the immunohistochemical findings in association with the clinical history and comparison of the morphology with the primary mediastinal tumor and its lung metastases established the final diagnosis of “brain metastatic atypical type A thymoma”.
Molecular profile of thymic tumor and lung metastases
DNA from formalin fixed paraffin embedded tissues obtained from the atypical type A component of mediastinal tumor and one of the lung metastases were isolated after microdissection with light microscope control using the NucleoSpin Tissue Kit (Macherey-Nagel, Düren., Germany) according to the manufacturer’s instructions. PCR was performed as described by Petrini et al. (10) using the following primer sequences: 5'-ATCCCGTACCCTCTTTTCC-3' (GTF2I_F) and 5'-AGACAAGAGTTCAACAGG-3' (GTF2I_R) for the first amplification round and 5'-AAGGAATTCCTTTTAGAAGG-3' (GTF2I_F1) and GTF2I_R for nested amplification. Both PCR conditions included an annealing temperature of 55 °C and 35 cycles of amplification. For nested PCR 1 µL of the first round amplification product was used. The L404H mutation was detected by Sanger sequencing using GTF2I_F1, GTF2I_R and the purified products from nested PCR. The sample of brain metastasis was unavailable at the time of the genetic analysis.
Discussion
Thymomas rarely (3–5%) metastasize beyond the thorax. Typically, thymoma metastases involve the pleura, while spreading to lymph nodes, lung and extrathoracic distant metastases is uncommon (11). The B2 and B3 morphologic types, higher stage and positive surgical margin are recognized as poor prognostic factors (11). Metastases to the CNS are exceedingly rare (12-14).
Our patient was finally diagnosed as AB type thymoma with an atypical type A component (according to WHO classification, 2015) and involvement of perithymic fat tissue. Although the histological type of AB thymoma is regarded as less aggressive, the stage was not high and surgical margin was negative, the distinct metastases to the lung and brain occurred after 10 and 15 years, respectively. Microscopically, the metastases resembled the atypical type A component of the primary tumor. The clinical relevance of atypical variant of the type A thymoma is unclear. Some authors suspect this subtype of being more aggressive than conventional (non-atypical) type A thymoma due to the common histological “markers of malignancy” like hypercellularity, nuclear atypia, increased mitotic and proliferative activity or necrosis (3,15). Vladislav et al. in their study on 23 cases of type A thymomas described that only necrosis in the tumor was a predictive factor of the progression of the disease (16). In our case, the morphology of the primary and metastatic tumors was similar but necrosis was not a constant feature. Bürger et al. analyzed genetic alterations in type A thymomas but did not find any differences between the conventional and atypical variants (17). However, the clinical history of our patient showed that only the atypical component revealed metastatic potential. This intriguing observation requires further investigation to determine its significance.
The thymoma in its typical, mediastinal location usually does not require any immunohistochemical studies. However, given the uncommon location of the metastasis, the brain, and the 15-year time period after the first resection a panel of immunohistochemistry was desirable to exclude other malignancies especially primary brain solitary fibrous tumor/hemangiopericytoma.
A molecular study of the sample from the atypical component of the primary tumor and one of metastases revealed the GTF2I (L404H) mutation detected by Sanger sequencing using GTF2I_F1, GTF2I_R and the purified products from nested PCR. This mutation is by far the commonest mutation of thymomas, with it being encountered in up to 80% of type A and type AB thymomas and only rarely in other types of these tumors (10). To the best of our knowledge this is the first description of a GTF2I mutation in atypical type A component of AB thymoma and its metastases.
The 5- and 10-year survival rates of patients with conventional type AB thymoma are high [estimated on 93–100% (14)] but in cases with brain metastasis the prognosis is significantly worse. It has been reported that the mean survival of thymic epithelial tumors with a single brain metastasis is about 256 days, whereas for multiple brain metastases it is only 64.4 days (18). No standard therapeutic approach to patients with metastatic thymoma has been established so far and it needs to be studied whether multimodal treatment, including neoadjuvant or adjuvant chemotherapy, surgery and postoperative radiotherapy may improve survival. Our patient was treated only by resection of primary and metastatic tumors and adjuvant radiotherapy after the first operation.
In conclusion, we report a rare case of an AB thymoma with atypical type A component (we propose the name “an atypical type AB thymoma”) that metastasized to the lung and to the brain after 10 and 15 years, respectively. The metastases exhibited histological features exclusively of the atypical type A component. In our opinion, the clinical history suggests the aggressive behavior of the atypical type A pattern and highlights the necessity of a careful microscopic examination of the AB thymomas for searching atypical areas. The atypical component may increase the risk of progression so extended follow-up for patients with such a diagnosis is required. We also propose the adding of an “atypical type AB thymoma” into the histological classification as a potentially more aggressive variant than conventional type AB thymoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The informed patient’s consent for publication could not be obtained because the patient died a year before the manuscript was written. It would be unusually burdensome to obtain the consent from the patient’s family because the contact with patient’s caregivers has been lost.
References
- Rosai J. World Health Organization: Histological Typing of the Thymus. 2nd ed Berlin, New York: Springer; 1999.
- Ströbel P, Marx A, Zettl A, et al. Thymoma and thymic carcinoma: an update of the WHO Classification 2004. Surg Today 2005;35:805-11. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IARC; 2015.
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Nonaka D, Rosai J. Is there a spectrum of cytologic atypia in type a thymomas analogous to that seen in type B thymomas? A pilot study of 13 cases. Am J Surg Pathol 2012;36:889-94. [Crossref] [PubMed]
- Vladislav IT, Gökmen-Polar Y, Kesler KA, et al. The prognostic value of architectural patterns in a study of 37 type AB thymomas. Mod Pathol 2014;27:863-8. [Crossref] [PubMed]
- Green AC, Marx A, Ströbel P, et al. Type A and AB thymomas: histological features associated with increased stage. Histopathology 2015;66:884-91. [Crossref] [PubMed]
- Marino M, Müller-Hermelink HK. Thymoma and thymic carcinoma. Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch A Pathol Anat Histopathol 1985;407:119-49. [Crossref] [PubMed]
- Kirchner T, Schalke B, Buchwald J, et al. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol 1992;16:1153-69. [Crossref] [PubMed]
- Petrini I, Meltzer PS, Kim IK, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 2014;46:844-9. [Crossref] [PubMed]
- Chalabreysse L, Roy P, Cordier JF, et al. Correlation of the WHO schema for the classification of thymic epithelial neoplasms with prognosis: a retrospective study of 90 tumors. Am J Surg Pathol 2002;26:1605-11. [Crossref] [PubMed]
- Kanayama S, Matsuno A, Nagashima T, et al. Symptomatic pituitary metastasis of malignant thymoma. J Clin Neurosci 2005;12:953-6. [Crossref] [PubMed]
- Ohata N, Usami N, Kawaguchi K, et al. Type AB thymoma with brain metastasis: Report of a case. Surg Today 2011;41:1436-8. [Crossref] [PubMed]
- Haryu S, Saito A, Inoue M, et al. Brain metastasis from invasive thymoma mimicking intracerebral hemorrhage: case report. Neurol Med Chir (Tokyo) 2014;54:673-6. [Crossref] [PubMed]
- Hashimoto M, Shimizu S, Takuwa T, et al. A case of atypical type A thymoma variant. Surg Case Rep 2016;2:116. [Crossref] [PubMed]
- Vladislav IT, Gökmen-Polar Y, Kesler KA, et al. The role of histology in predicting recurrence of type A thymomas: a clinicopathologic correlation of 23 cases. Mod Pathol 2013;26:1059-64. [Crossref] [PubMed]
- Bürger T, Schaefer IM, Küffer S, et al. Metastatic type A thymoma: morphological and genetic correlation. Histopathology 2017;70:704-710. [Crossref] [PubMed]
- Ersahin M, Kilic K, Gögüsgeren MA, et al. Multiple brain metastases from malignant thymoma. J Clin Neurosci 2007;14:1116-20. [Crossref] [PubMed]


