Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation: a randomized controlled trial
Introduction
Fluid management is an important issue for patients during surgery. The intraoperative fluid infusion balance between hypovolemia and overload has been identified as a major contributing factor to avoid the postoperative complications, such as systemic inflammatory response syndrome, ileus, increased cardiac demands, and even multiple organ failure (1-3). The conventional fluid management is based on the clinical signs such as mean arterial pressure (MAP), central venous pressure (CVP), or urine output that are only slightly related to the hemodynamic goals of fluid administration (1). Previous studies indicated that fluid titration based on the measurements of functional hemodynamic variables, such as stroke volume variation (SVV), which was obtained by pulse contour analysis and variation of stroke volume during the respiratory cycle, was useful to exert a superior effect in improving end-organ perfusion and oxygenation. Similarly, it can also decrease the rate of postoperative complications in different surgical patients (4-8).
One-lung ventilation (OLV), a non-physiological ventilation approach, is widely used in thoracic surgery. Previous studies revealed that the incidence of postoperative acute lung injury (ALI) is 2–5% after major thoracic surgery (9). Although the exact pathogenesis of OLV-related ALI undergoing lobectomy has not been fully elucidated, previous studies have indicated that the intraoperative oxygenation dysfunction and overload fluid management during thoracic surgery has been identified as risk factors for lung injuries and other pulmonary complications such as pulmonary edema (10-13). For specific surgical procedures, reducing the amount of intraoperative fluid infusion and improving oxygenation is of paramount importance in thoracic surgery. Nevertheless, it is well known that the blinded or uncontrolled fluid restriction may cause other hypovolemia-related complications, such as tissue hypoxia, which play a potent role in organ dysfunction and increased postoperative morbidity and mortality (14,15).
Previous studies have demonstrated that SVV is useful to predict fluid responsiveness legitimately during OLV with acceptable levels of sensitivity and specificity (16,17). However, studies exploring the effect of intraoperative fluid restriction protocol based on advanced hemodynamic parameters on patients’ oxygenation and postoperative prognosis during OLV are yet lacking. In the present study, we hypothesized that the use of cardiovascular measurements might be valuable in striking a balance between the risks of insufficient and excessive fluid intake. Therefore, we designed an intraoperative goal-directed fluid restriction (GDFR) protocol where minimal fluid maintenance according to the recent literature in patients during thoracic surgery (18).
The aim of the clinical study was to investigate the effect of intraoperative GDFR protocol using SVV and cardiac index on intrapulmonary oxygenation and postoperative outcomes in patients undergoing OLV. The primary outcome variable was PaO2/FiO2, a useful variable for detecting impaired intrapulmonary oxygenation and gas exchange (19,20). The secondary outcomes were other pulmonary variables and pneumodynamics, inflammatory response, and the incidence of postoperative pulmonary complications.
Methods
Patients
Between April 2016 and February 2017, 180 adult patients diagnosed with primary non-small-cell lung cancer with a preoperative clinical stage IA or IB as assessed by computer tomography or positron emission tomography-computed tomography scan were scheduled for thoracoscopic lobectomy undergoing OLV, and those that satisfied the following inclusion criteria were recruited: aged between 18 and 60 years; American Society of Anesthesiologists physical (ASA) status I-II category; body mass index between 18.5 and 25kg/m2. The exclusion criteria included severe impairment of renal and cardiac function (New York Heart Association classes III-IV); Preoperative abnormal lung function (forced expiratory volume in 1 s <50% of the predicted values); systemic or local active infections (clinically defined, leukocytosis, or the body temperature >38 °C); Preoperative acid-base or electrolyte imbalance; Intraoperative frequent cardiac arrhythmias and OLV time <60 min.
Randomization and masking
All the enrolled patients were allocated in a 1:1 ratio to undergo fluid management during OLV into either the GDFR protocol group (group G) or the conventional fluid management group (group C). The randomization was stratified by sequential blocking based on the computer random number generator. Allocations details were kept in sealed envelopes marked by serial number. Before the induction of anesthesia, the sealed, numbered and opaque envelopes containing the treatment assignments were opened by an independent anesthesiologist. The data assessment or analysis was performed by an independent research staff supervised by an independent statistician. To make sure the reliability of data acquisition, the patients, the clinical researchers for the collection of data and blood samples and postoperative follow-up team were all blinded to group allocation. Group allocation was scarcely revealed when the final data analysis was completed.
Anesthesia and monitoring
The patients received a restricted diet before surgery. Before the induction of anesthesia, the standardized central venous puncture was performed, and a 20-G arterial line (B. Braun Medical Inc., Bethlehem, PA, USA) was inserted into the radial artery of the nondominant forearm. The heart rate (HR), MAP, CVP, pulse oxygen saturation (SpO2), end-tidal carbon dioxide partial pressure (PETCO2), temperature, and bispectral index (BIS) were continuously monitored on a multifunction screen (Philips Medizin, Hamburg, Germany). The SVV, cardiac index (CI), cardiac output, and stroke volume were measured by the FloTrac/Vigileo system (Edwards Lifesciences, Irvine, CA, USA).
General anesthesia was initiated with intravenous 0.05 mg/kg midazolam, 2 mg/kg propofol, 0.4 mg/kg sufentanil, and 1.0 mg/kg rocuronium. Subsequently, the intraoperative anesthesia was maintained with a continuous infusion of propofol (4–8 mg/kg/h) and remifentanil (0.05–0.2 µg/kg/min) in order to achieve a target BIS value between 40 and 50. After the induction of anesthesia, a left-sided double-lumen endobronchial tube (Mallinckrodt, Dublin, Ireland) was inserted and confirmed by bronchoscopy. Patients were ventilated in volume controlled mode by using an anesthesia machine (S/5 Avance, Datex-Ohmeda, Madison, USA) following the protocol with tidal volume (VT) 8 mL/kg (two-lung ventilation) or 6 mL/kg (OLV), fraction of inspired oxygen (FiO2) 100%, inspiratory to expiratory time (I/E) ratio 1:2, and positive end-expiratory pressure (PEEP) 5 cmH2O; the respiratory rates were adjusted to maintain the PETCO2 between 35 and 45 mmHg. The continuous positive airway pressure (CPAP) of 1–2 cmH2O to the nonventilated lung or recruitment maneuver to the ventilated lung was applied for a limited period to the non-dependent lung when the peripheral saturation decreased below 90%. The nasopharyngeal temperature was maintained >36 °C by fluid warming devices or medical warming blankets. The intermittent additional sufentanil or cisatracurium was administered during surgery as per the requirement.
Intervention protocol
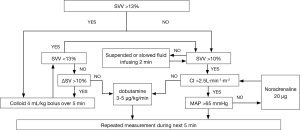
All the practitioners in this study were experienced with the FloTrac/Vigileo device. In both groups, the intraoperative basal fluid replacement was achieved by continuous infusion of the 4 mL/kg/h crystalloid solution after general anesthesia induction. For group C, the anesthesiologist administered additional fluids in those who underwent conventional fluid management according to the principles of Miller’s Anesthesia or used vasoactive substances, if necessary, aiming at MAP >65 mmHg, HR 60–100 bpm, CVP 6–12 mmHg, and the urine output >0.5 mL/kg/h. Patients in group G received the intraoperative fluid management during OLV, and the protocol was summarized in Figure 1. In the case that SVV was >13%, 4 mL/kg bolus of colloid (hydroxyethyl starches 130/0.4 in 6%, Fresenius Kabi AG, Bad Homburg, Germany) infused over 5 min was administered, and if the SV increased by more than 10%, the bolus was repeated until SVV <13%. In the case that SVV was <10%, then the bolus was suspended or infused with a slow speed in order to maintain SVV >10%. An infusion of dobutamine 3–5 µg/kg/min was administered after the SV failed to increase by more than 10% or CI less than 2.5 L/min/m2. The intravenous norepinephrine bolus of 20 µg was allowed when the fluid infusion failed to maintain the MAP >65 mmHg. The hemodynamic status was repeatedly measured during the next 5 min.
The postoperative complications were recorded in both groups after the end of surgery. The volume of totally administered crystalloid and colloid, blood loss, urine volume, and the requirement for vasoactive agents was recorded and analyzed. The threshold of transfusion with packed red blood cells was set at the hemoglobin value <8 g/dL or hematocrit <25%.
Blood samples
The radial arterial blood samples were collected for analysis before OLV (T0, baseline), 30 min (T1) and 60 min (T2) after OLV, 10 min after re-expansion (T3), and the end of the operation (T4) using a blood gas system (Roche Diagnostics GmbH, Mannheim, Germany). The venous blood was sampled from the central venous at T0, T4, and 6 h (T5), 24 h (T6), and 72 h (T7) after the operation, and centrifuged at 2,000 rpm for 15 min at 4 °C. The collected serum samples were immediately preserved at −80 °C for subsequent analysis.
Lung function and Qs/Qt ratio
The lung function evaluation including PaO2/FiO2, alveolar to the arterial difference of oxygen tension (A-aDO2), and respiratory index (RI = A-aDO2/PaO2) was determined according to the result of the blood gas analysis at the above time-points. The peak airway pressure (Ppeak), plateau airway pressure (Pplat), and dynamic lung compliance (Cdyn) of individual patients at T0–T4 were monitored and obtained directly from the ventilator setting. The Qs/Qt ratio was calculated from the following formula: (CCO2 − arterial oxygen content)/(CCO2 − mixed venous oxygen content), the CCO2 represents the end-pulmonary capillary oxygen content (21).
Postoperative pulmonary-related adverse events such as pneumothorax, pneumonia, pulmonary edema, ALI, and acute respiratory distress syndrome (ARDS) during hospitalization were confirmed based on the chest X-ray characteristics and laboratory examinations according to the American-European Consensus Conference on ARDS guidelines (22).
Inflammatory response
The concentrations of the serum cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10) were measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (R&D, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Short-term outcomes
The length of hospital stay, cardiovascular complications such as hypotension or heart failure (increased cardiac enzyme and NT-proBNP). If age less than 50: NT-proBNP >450 ng/L; if age more than 50: NT-proBNP >900 ng/L), renal dysfunction (creatinine >180 µmol/L) or renal failure (creatinine >450 µmol/L), and gastrointestinal complications after surgery were recorded.
Statistical analysis
To calculate the sample size, we considered a difference of 20 mmHg for PaO2/FiO2 between the two groups as reported previously (19). A standard deviation (SD) of 50 mmHg of the means, with a one-sided type I error of 0.05 and compensating 10% cases for potential dropouts; thus, a minimum sample size of 90 patients in each group allowed an 80% statistical power for enrollment in the study.
The statistical analysis was performed using SPSS 16.0 software. Data were assessed for normality using the Kolmogorov-Smirnov test; the continuous normally distributed data were assessed with the mean ± SD and compared using the independent t-test. The skewed data were presented as median (range) and compared using the Mann-Whitney U test or Wilcoxon rank-sum test for unpaired and paired results respectively. The categorical data were presented as frequency or percentage and compared using the Fisher’s exact test. The concentrations of serum markers were analyzed by repeated-measures ANOVA using Bonferroni correction for post hoc analysis. A P-value less than 0.05 was considered statistically significant.
Results
Patient demographics
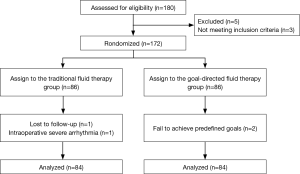
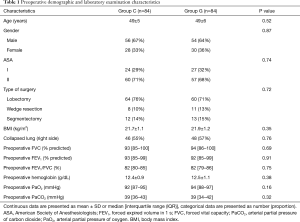
A total of 180 patients were assessed for inclusion eligibility in this study. Despite preoperative approval, 5 patients were excluded from the data analysis, 3 patients were not meeting inclusion criteria, 1 patient was lost to follow-up, 1 patient had an intraoperative severe arrhythmia, and 2 patients were failed to achieve predefined goals. Thus, 168 were ultimately recruited for analysis and randomly assigned to the GDFR group (group G, n=84) or the conventional fluid therapy group (group C, n=84) as shown in the Figure 2. The preoperative demographic and laboratory examination characteristics were summarized in Table 1. No statistically significant differences were observed between the two groups with respect to their baseline characteristics. None of the patients experienced adverse surgical events throughout the intraoperative period.
Full table
Blood gas analysis, lung mechanics and functions
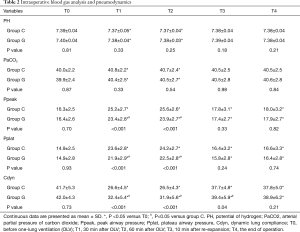
The intraoperative blood gas analysis and lung mechanics were summarized in Table 2. In comparison with the baseline, pH decreased significantly, whereas PaCO2 increased in both groups during OLV. The Ppeak and Pplat in group G were lower than those in group C, whereas the values of Cdyn were higher than those in group G from T1 to T3 with a significant difference between the groups during OLV.
Full table
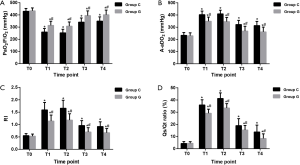
As shown in Figure 3, the baseline values of PaO2/FiO2, A-aDO2, RI, and Qs/Qt ratio did not differ baseline between the groups. The values of PaO2/FiO2 were distinctly decreased (all P<0.01), whereas the A-aDO2, RI, and Qs/Qt ratio increased significantly in both groups after OLV was established with significant differences between the groups (all P<0.05).
Intraoperative hemodynamic data
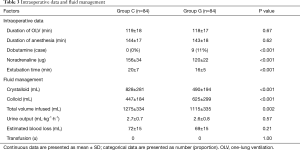
The intraoperative hemodynamic measures, fluid managements and vasoactive drug uses were summarized in Table 3. No statistically significant differences were observed in the duration of OLV and anesthesia between the two groups, whereas the extubating period was significantly earlier in the GDFR group (P<0.01). Dobutamine was used in 9 patients from group G when the CI was less than 2.5 L/min/m2; however, the bolus of noradrenaline usage in group G was less than that in group C (P<0.01). None of the patients received intraoperative blood transfusion; the total volume of intravenous infusion and crystalloids in group G was significantly lower than that in group C, whereas the volume of colloids was significantly higher than that in group C (all P<0.01). No differences were seen in intraoperative blood losses and urine outputs between the two groups.
Full table
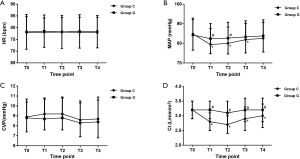
As shown in Figure 4, The HR and CVP were similar between the two groups at all observational time-points. However, compared to the baseline, the values of MAP were decreased significantly with the initiation of OLV but before the end of surgery in group C. In addition, the values of MAP in group G were greater than those in group C at T1 (P=0.02) and T2 (P=0.03). On the other hand, the values of CI in group C were decreased after OLV begun, and a significant difference was observed between the groups (all P<0.01).
Inflammatory cytokines
As shown in Figure 5, no statistically significant differences were observed in the concentrations of serum TNF-α, IL-6, and IL-10 between the two groups at baseline. At later observational time points, these levels were significantly increased as compared to the baseline. In addition, the IL-6 levels peaked at 24 h after the operation, whereas that of TNF-α and IL-10 peaked at 6 h after the operation. However, all of the three did not exhibit any significant difference between groups at obvious time-points.

Short-term outcomes
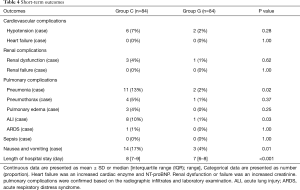
The short-term outcomes were summarized in Table 4. The postoperative incidences of pneumonia, ALI, and nausea and vomiting in group G were lower than those in group C (P=0.02, 0.03, 0.01, respectively). Additionally, the length of hospital stay in group G was significantly lower than those in group C (P<0.01).
Full table
Discussion
The principal findings of the clinical study are that SVV-based GDFR protocol improves intraoperative pulmonary oxygenation, lowering the airway pressure and increasing the dynamic lung compliance during surgery. Furthermore, the GDFR protocol stabilized the intraoperative hemodynamics efficiently. Importantly, it can also reduce the length of hospital stay, postoperative nausea and vomiting, and the incidence of postoperative pulmonary complications such as pneumonia and ALI. To the best of our knowledge, this is the first study indicating the protective effect of SVV-based GDFR protocol using FloTrac/Vigileo system on the lung function during OLV.
For patients during thoracic surgery, the purpose of GDFR protocol is not only to reduce the amount of intraoperative fluid infusion, it also wants to optimize the end-organ perfusion and stabilize the hemodynamic status with suitable fluids according to the dynamic parameters. With the chest open via surgical procedures, much of the pressure generated by the ventilator would not be transmitted to the pulmonary vessels but rather to the atmosphere, which may result in a decrease in SVV. However, the ventilated lung is actually not open to the atmosphere because its pleura are still intact and the mediastinum also separates that lung from the atmosphere (16). Thus, we believe that SVV could be predictive of fluid responsiveness during OLV. Previous research shown that optimal threshold value of SVV to discriminate between fluid responders and non-responders during OLV was more than 10% (16,17). On the other hand, SVV less than 13% identifies fluid responder patients with high sensitivity and specificity (23,24). Therefore, we decided to use a high cut-off value of SVV between 10% to 13%, in order to retain minimal fluid and maintain the patients on the “dry” side as much as possible. Meanwhile, in the present study, this protocol states that a minimum threshold value of CI >2.5 L/min/m2 ensures an adequate supply of oxygen to the tissues (25).
In the current study, the indicators including PaO2/FiO2, A-aDO2 and RI were chosen as the primary variables to assess the intraoperative lung function due to its efficiency in reflecting intrapulmonary oxygenation and gas exchange (19,26-28). Compared with traditional fluid management, our findings reveal a positive effect of GDFR protocol that alleviate the decreased arterial oxygen content and intrapulmonary gas exchange. Li et al. (29) have demonstrated that GDFR protocol could reduce the pulmonary vascular resistance and protect the right ventricular dynamic function in patients with severe pulmonary arterial hypertension. Similarly, we also reveal that GDFR protocol is useful to improve intrapulmonary shunt and decrease venous admixture. Thus, we consider that the possible mechanisms to improve intrapulmonary oxygenation and gas exchange by GDFR protocol is alleviating the pulmonary vascular resistance and minimizing the hydrostatic pressure.
It is well known that mechanical disruption of the alveolar capillary barrier obviously contributes to the lung injury (30). Indeed, rapid fluid infusion might damage the underlying endothelial cells, alveolar epithelial cells, and the surfactant, which may increase barrier permeability, and reduce the alveolar epithelial fluid clearance from the air spaces (31,32). Kapoor et al. (33) have provided evidence supporting the effect of GDFR protocol in decreasing the content of extravascular lung water. In the present study, there is a significant improvement in lung mechanics with GDFR protocol, which is potential via alleviating epithelial and endothelial permeability and decreasing pulmonary edema.
The inflammatory response is demonstrated to play a vital role in the pathogenesis of lung injury during OLV (34). However, in this study, we found that patients prescribing to an SVV-guided fluid regimen did not experience a reduction in both pro- and anti-inflammatory response, thereby demonstrating that GDFR protocol is not valuable in adjusting the local or systemic inflammation during OLV, which were somewhat similar to those of Funk et al. (35) and Fitzgerald et al. (36).
On the other hand, using a crystal liquid supplement may retain most of the crystals in the blood vessels. Besides, hydroxyethyl starch solution was found to be more likely to maintain gastrointestinal microcirculation perfusion and oxygen tension than the crystal (37,38). we therefore decide to use colloid instead of crystalloid in the GDFR protocol. Although it is not always ideal to use a colloidal solution, as anesthesiologists need to consider various factors, such as nephrotoxic effects (39). However, in the present study, there was no significant difference in the urine volume and postoperative renal complications, which suggested that the bolus of hydroxyethyl starch solution we used not exceed the kidney compensatory. The results of our study also shown that patients undergoing GDFR protocol received a significantly lower amount of intravenous infusion with a different quality of colloids and crystalloids. Meanwhile, the use of vasoactive drugs was also different between groups, which may be the results of different liquid treatment program between groups. Thus, we believe that different types and dose of fluid and vasoactive drugs with the accurate opportunity may prevent the administration of excessive fluid.
Nonetheless, our study harbors several limitations. First, due to the unwillingness of some patients, our study was not evaluated the postoperative intrapulmonary gas exchange and oxygenation based on arterial blood gas. In addition, the long-term effects of SVV-based GDFR protocol on lung function were also lost to follow-up. Second, although we were attempted to exclude the potential influencing factors, some other factors such as social and genetics may continue to interfere the accuracy of the results. Third, this is a single-center research, hence, a multicenter study may alleviate the investigation with respect to the potential of GDFR. Fourth, it is difficult for us to measure the postoperative ARDS based on newer Berlin definition without PEEP after extubation. Therefore, we use the old guidelines of ARDS based on American-European Consensus Conference.
Conclusions
In summary, the current study showed that SVV-based GDFR protocol applied to the patients undergoing OLV might improve the intraoperative pulmonary oxygenation. Moreover, SVV-based GDFR protocol can also reduce the postoperative complications and the length of hospital stay; however, it is not useful in reducing the local or systemic inflammation. Nevertheless, these findings necessitate validation based on a multicenter study.
Acknowledgements
We appreciated Dr. Shan-Shan Hu for developing statistical analysis plan and preparing the manuscript.
Funding: This research was supported by the Nation Natural Science Foundation of China (No.81503080), the Anhui Provincial Natural Science Foundation (No.1608085QH210), the Anhui Provincial Application Research of Public Welfare Technology on Linkage Projects (1604f0804019).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This prospective, single-center, randomized clinical study protocol was approved by the Chinese Clinical Trial Registry (No. ChiCTR–IPR-16008288) and the Ethics Committee of Anhui Medical University (Hefei, China). After ethics was approved, all patients were informed of the entire procedure and written informed consents were obtained after a detailed explanation of the study. During the process of research, all treatments were carried out in accordance with the Declaration of Helsinki and the Use Committee of Affiliated Provincial Hospital of Anhui Medical University.
References
- Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 2015;41:1529-37. [Crossref] [PubMed]
- Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311:2181-90. [Crossref] [PubMed]
- Peng K, Li J, Cheng H, et al. Goal-directed fluid therapy based on stroke volume variations improves fluid management and gastrointestinal perfusion in patients undergoing major orthopedic surgery. Med Princ Pract 2014;23:413-20. [Crossref] [PubMed]
- Azhar RA, Bochner B, Catto J, et al. Enhanced Recovery after Urological Surgery: A Contemporary Systematic Review of Outcomes, Key Elements, and Research Needs. Eur Urol 2016;70:176-87. [Crossref] [PubMed]
- Benes J, Giglio M, Brienza N, et al. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care 2014;18:584. [Crossref] [PubMed]
- Giglio MT, Marucci M, Testini M, et al. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 2009;103:637-46. [Crossref] [PubMed]
- Mayer J, Boldt J, Mengistu AM, et al. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care 2010;14:R18. [Crossref] [PubMed]
- Osawa EA, Rhodes A, Landoni G, et al. Effect of Perioperative Goal-Directed Hemodynamic Resuscitation Therapy on Outcomes Following Cardiac Surgery: A Randomized Clinical Trial and Systematic Review. Crit Care Med 2016;44:724-33. [PubMed]
- Licker M, Fauconnet P, Villiger Y, et al. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol 2009;22:61-7. [Crossref] [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91; discussion 91. [Crossref] [PubMed]
- Arslantas MK, Kara HV, Tuncer BB, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J Thorac Cardiovasc Surg 2015;149:314-20, 321.e1.
- Baudouin SV. Lung injury after thoracotomy. Br J Anaesth 2003;91:132-42. [Crossref] [PubMed]
- Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [Crossref] [PubMed]
- Kertai MD, Klein J, Bax JJ, et al. Predicting perioperative cardiac risk. Prog Cardiovasc Dis 2005;47:240-57. [Crossref] [PubMed]
- Thacker JK, Mountford WK, Ernst FR, et al. Perioperative Fluid Utilization Variability and Association With Outcomes: Considerations for Enhanced Recovery Efforts in Sample US Surgical Populations. Ann Surg 2016;263:502-10. [Crossref] [PubMed]
- Suehiro K, Okutani R. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing one-lung ventilation. J Cardiothorac Vasc Anesth 2010;24:772-5. [Crossref] [PubMed]
- Zhang J, Chen CQ, Lei XZ, et al. Goal-directed fluid optimization based on stroke volume variation and cardiac index during one-lung ventilation in patients undergoing thoracoscopy lobectomy operations: a pilot study. Clinics (Sao Paulo) 2013;68:1065-70. [Crossref] [PubMed]
- Luo J, Xue J, Liu J, et al. Goal-directed fluid restriction during brain surgery: a prospective randomized controlled trial. Ann Intensive Care 2017;7:16. [Crossref] [PubMed]
- Li C, Xu M, Wu Y, et al. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: a randomized controlled study. Anesthesiology 2014;121:249-59. [Crossref] [PubMed]
- Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2005;172:206-11. [Crossref] [PubMed]
- Chai XQ, Ma J, Xie YH, et al. Flurbiprofen axetil increases arterial oxygen partial pressure by decreasing intrapulmonary shunt in patients undergoing one-lung ventilation. J Anesth 2015;29:881-6. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010;14:R118. [Crossref] [PubMed]
- Marik PE, Cavallazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009;37:2642-7. [Crossref] [PubMed]
- Colantonio L, Claroni C, Fabrizi L, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg 2015;19:722-9. [Crossref] [PubMed]
- Alexiou C, Tang AA, Sheppard SV, et al. The effect of leucodepletion on leucocyte activation, pulmonary inflammation and respiratory index in surgery for coronary revascularisation: a prospective randomised study. Eur J Cardiothorac Surg 2004;26:294-300. [Crossref] [PubMed]
- Cressoni M, Caironi P, Polli F, et al. Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med 2008;36:669-75. [Crossref] [PubMed]
- Tedjasaputra V, Bryan TL, van Diepen S, et al. Dopamine receptor blockade improves pulmonary gas exchange but decreases exercise performance in healthy humans. J Physiol 2015;593:3147-57. [Crossref] [PubMed]
- Li S, Ma Q, Yang Y, et al. Novel Goal-Directed Hemodynamic Optimization Therapy Based on Major Vasopressor during Corrective Cardiac Surgery in Patients with Severe Pulmonary Arterial Hypertension: A Pilot Study. Heart Surg Forum 2016;19:E297-302. [Crossref] [PubMed]
- Frank JA, Wray CM, McAuley DF, et al. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L1191-8. [Crossref] [PubMed]
- Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 2010;87:320-30. [Crossref] [PubMed]
- Ware LB, Fremont RD, Bastarache JA, et al. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J 2010;35:331-7. [Crossref] [PubMed]
- Kapoor PM, Magoon R, Rawat RS, et al. Goal-directed therapy improves the outcome of high-risk cardiac patients undergoing off-pump coronary artery bypass. Ann Card Anaesth 2017;20:83-9. [Crossref] [PubMed]
- Luo S, Wang Y, An Q, et al. Platelets protect lung from injury induced by systemic inflammatory response. Sci Rep 2017;7:42080. [Crossref] [PubMed]
- Funk DJ. A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Crit Care 2015;19:247. [Crossref] [PubMed]
- Fitzgerald DC, Holmes SD, St Onge JR, et al. Systemic inflammatory response during cardiac surgery: a pilot study. Innovations (Phila) 2015;10:125-32. [Crossref] [PubMed]
- Hiltebrand LB, Kimberger O, Arnberger M, et al. Crystalloids versus colloids for goal-directed fluid therapy in major surgery. Crit Care 2009;13:R40. [Crossref] [PubMed]
- Kimberger O, Arnberger M, Brandt S, et al. Goal-directed colloid administration improves the microcirculation of healthy and perianastomotic colon. Anesthesiology 2009;110:496-504. [Crossref] [PubMed]
- Vives M, Callejas R, Duque P, et al. Modern hydroxyethyl starch and acute kidney injury after cardiac surgery: a prospective multicentre cohort. Br J Anaesth 2016;117:458-63. [Crossref] [PubMed]