The Lotus dilemma—respond to paravalvular leakage, but not answering pacemaker implantations?
The treatment of symptomatic aortic valve stenosis has been revolutionized by transcatheter aortic valve replacement (TAVR), evolving from a last resort therapy to the standard treatment of patients at intermediate or high risk for conventional surgery (1,2). The continuous evolution of transcatheter heart valves (THV) and delivery systems along with a considerable increase in operator experience has led to a steady improvement of outcomes with a 30-day mortality approaching the 1% threshold. Continuous iterations of balloon-expandable and self-expanding devices as well as the pursue of alternative implantation technologies to address limitations of earlier generations such as paravalvular leakage (PVL), requirement for new permanent pacemaker implantations (PPI), and vascular complications have been performed.
Among alternative implantation technologies, the Lotus Valve SystemTM (Boston Scientific, Marlborough, Massachusetts, USA) is the first commercially available THV, employing a technology best described as mechanical expansion. This device consists of a braided single-wire nitinol frame with three bovine pericardial leaflets and features an adaptive polymer membrane seal at the lower half to reduce PVL. The Lotus valve is available in three sizes (23, 25, and 27 mm) and is delivered via an 18-F (for the 23 mm size) and 20-F introducer (for the 25 and 27 mm sizes). Guided by a tantalum marker, the device is mechanically expanded in the desired position and allows for full reposition after careful evaluation of the initial result and is locked after reaching the final position.
The Lotus valve system has been studied in a considerable number of studies most of which included small to intermediate-sized populations. The REpositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus™ Valve System—Randomized Clinical Evaluation (REPRISE)-I study (3) was an early feasibility trial including 11 patients and laid out the path for the larger REPRISE-II study, which eventually led to CE-approval of the device (4,5). In REPRISE-II, 120 high-risk patients from Europe and Australia were included, where a 30-day and a 1-year mortality of 4.2% and 10.9% were found, respectively. With only 1%, the rate of moderate to severe PVL was very low. After completion, the REPRISE-II study was extended to a cohort of 250 patients and was published as REPRISE-IIE (6). In this extended cohort, PVL rate was also remarkably low with 0.6% of the patients displaying moderate to severe PVL. Nevertheless, despite these very promising achievements in the near abolishment of PVL, the REPRISE program also clarifies a major problem of this novel THV: the need of PPI in over 30% of pacemaker naive patients. This worrisome PPI rate has been confirmed by numerous independent groups with PPI rates ranging from 24–38% (7-10). A summary of selected studies on the Lotus valve system is displayed in Figure 1. Prompted by these findings, considerable effort has been invested to elucidate the underlying mechanisms of this elevated PPI rate of the Lotus valve system. In a study by Rampat et al., excluding patients with a pacemaker at baseline, new PPI rate was 32% and was only predicted by baseline conduction abnormalities (11). In a recent subanalysis of the REPRISE IIE trial, implantation technique (implantation depth >5 mm) and annular oversizing (defined as LVOT overstretch of >10%) were identified as predictors for new PPI (6).
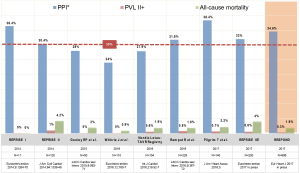
The recently published REpositionable Lotus Valve System—POst-Market EvaluatioN of Real WorlD Clinical Outcomes (RESPOND) study (12), is a prospective, open-label, single-arm, multicenter study, which assesses the safety and efficacy of the Lotus THV in routine clinical practice in a large cohort of patients. Of 1014 patients included in the study, 996 patients received the Lotus THV and were included in the as-treated population. In almost 30%, repositioning of the Lotus THV was successfully attempted. As expected from the intermediate risk patients and the remarkable expertise of participating operators, 30-day all-cause mortality and disabling stroke rate were 2.6% and 2.2%, respectively. Further, this study confirms results from smaller, earlier trials with a very low rate of PVL (0.3%) and a very high rate of PPI (34.6%).
In the case of the Lotus THV, it becomes clear that there seems to exist a dilemma between the achievement of low PVL rates at the expense of PPI. With PVL rates ranging between 0-1%, the Lotus THV has taken the lead in the field among competitors, such as SAPIEN 3, Evolut R and ACURATE neo, having PVL II+ rates of 2.6–3.7% (13,14), 1.9–5.3% (15-17) and 4.1–4.8% (18), respectively. PPI rates of several “next-generation” THVs are considerably different. It ranges from around 10% with Symetis ACURATE neo (18), 12–13% with SAPIEN 3 (13,14), 15–20% with Evolut R (15-17) and about 30% with the Lotus valve system (12).
Although in earlier studies moderate or severe PVL has been linked to poorer outcomes after TAVR (19,20), the impact of PPI on long-term outcome remains controversial. While no difference has been observed in earlier studies (21) recent data from the PARTNER consortium has revealed chronic pacing as a predictor of mortality (22,23). Therefore, it is important to note that RESPOND will provide us with long-term follow-up to clarify the prognostic impact of PVL and PPI in patients treated with Lotus valve system.
Additionally, among other reasons, elevated PPI rates have prompted the development of the next generation of Lotus valve system, Lotus Edge which has received CE-Mark in September 2016. In comparison to the Lotus valve system, this next iteration provides a more flexible and lower profile catheter equipped with Depth Guard™, a feature to prevent deep implantation in order to reduce new PPI rate.
The ongoing REPRISE-III trial (NCT02202434) has recently completed enrolment and constitutes a head-to-head randomized controlled comparison of Lotus/Lotus Edge versus CoreValve/Evolut R in a 2:1 fashion. This trial includes 1,032 patients in up to 60 centers in North America, Europe and Australia and will further elucidate the comparative effectiveness of the Lotus valve system and will also include new insights on Lotus Edge.
However, as of today the Lotus valve system has been recalled from the market due to problems with the devices locking system resulting in excess tension in the pin mechanism and is expected to be back on the market in the last quarter of 2017.
In conclusion, the RESPOND trial showed that the Lotus THV can be safely used in routine daily practice. With a favorable safety profile and the lowest PVL rate to date, but quite high new PPI rates, the comparative effectiveness of the Lotus valve system needs to be determined in direct randomized comparison, such as the recently completed REPRISE-III trial. Long-term follow-up after Lotus implantation is warranted in order to assess the prognostic impact of the elevated PPI rate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Meredith IT, Worthley SG, Whitbourn RJ, et al. Transfemoral aortic valve replacement with the repositionable Lotus Valve System in high surgical risk patients: the REPRISE I study. EuroIntervention 2014;9:1264-70. [Crossref] [PubMed]
- Meredith Am IT, Walters DL, Dumonteil N, et al. Transcatheter Aortic Valve Replacement for Severe Symptomatic Aortic Stenosis Using a Repositionable Valve System: 30-Day Primary Endpoint Results From the REPRISE II Study. J Am Coll Cardiol 2014;64:1339-48. [Crossref] [PubMed]
- Meredith IT, Walters DL, Dumonteil N, et al. 1-Year Outcomes With the Fully Repositionable and Retrievable Lotus Transcatheter Aortic Replacement Valve in 120 High-Risk Surgical Patients With Severe Aortic Stenosis: Results of the REPRISE II Study. JACC Cardiovasc Interv 2016;9:376-84. [Crossref] [PubMed]
- Dumonteil N, Meredith IT, Blackman DJ, et al. Insights into the Need for Permanent Pacemaker Following Implantation of the Repositionable LOTUS™ Valve for the Transcatheter Aortic Valve Replacement in 250 Patients: Results from the REPRISE II Trial With Extended Cohort. EuroIntervention 2017. [Epub ahead of print]. [PubMed]
- Gooley RP, Talman AH, Cameron JD, et al. Comparison of Self-Expanding and Mechanically Expanded Transcatheter Aortic Valve Prostheses. JACC Cardiovasc Interv 2015;8:962-71. [Crossref] [PubMed]
- Wöhrle J, Gonska B, Rodewald C, et al. Transfemoral aortic valve implantation with the repositionable Lotus valve for treatment of patients with symptomatic severe aortic stenosis: results from a single-centre experience. EuroIntervention 2016;12:760-7. [Crossref] [PubMed]
- De Backer O, Götberg M, Ihlberg L, et al. Efficacy and safety of the Lotus Valve System for treatment of patients with severe aortic valve stenosis and intermediate surgical risk: Results from the Nordic Lotus-TAVR registry. Int J Cardiol 2016;219:92-7. [Crossref] [PubMed]
- Pilgrim T, Stortecky S, Nietlispach F, et al. Repositionable Versus Balloon-Expandable Devices for Transcatheter Aortic Valve Implantation in Patients With Aortic Stenosis. J Am Heart Assoc 2016;5:e004088. [Crossref] [PubMed]
- Rampat R, Khawaja MZ, Hilling-Smith R, et al. Conduction abnormalities and permanent pacemaker implantation after transcatheter aortic valve replacement using the repositionable LOTUS device: the United Kingdom experience. JACC Cardiovasc Interv 2017;10:1247-53. [Crossref] [PubMed]
- Falk V, Wöhrle J, Hildick-Smith D, et al. Safety and efficacy of a repositionable and fully retrievable aortic valve used in routine clinical practice: the RESPOND Study. Eur Heart J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Wendler O, Schymik G, Treede H, et al. SOURCE 3: 1-year outcomes post-transcatheter aortic valve implantation using the latest generation of the balloon-expandable transcatheter heart valve. Eur Heart J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J 2016;37:2252-62. [Crossref] [PubMed]
- Popma JJ, Reardon MJ, Khabbaz K, et al. Early Clinical Outcomes After Transcatheter Aortic Valve Replacement Using a Novel Self-Expanding Bioprosthesis in Patients With Severe Aortic Stenosis Who Are Suboptimal for Surgery: Results of the Evolut R U.S. Study. JACC Cardiovasc Interv 2017;10:268-75. [Crossref] [PubMed]
- Kalra SS, Firoozi S, Yeh J, et al. Initial Experience of a Second-Generation Self-Expanding Transcatheter Aortic Valve: The UK & Ireland Evolut R Implanters' Registry. JACC Cardiovasc Interv 2017;10:276-82. [Crossref] [PubMed]
- Grube E, Van Mieghem NM, Bleiziffer S, et al. Clinical out-comes with a repositionable self-expanding trans-catheter aortic valve prosthesis: the international FORWARD Study. J Am Coll Cardiol 2017;70:845-53. [Crossref] [PubMed]
- Husser O, Kim WK, Pellegrini C, et al. Multicenter comparison of novel self-expanding versus balloon-expandable transcatheter heart valves. J Am Coll Cardiol Intv 2017. In press.
- Kodali S, Pibarot P, Douglas PS, et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J 2015;36:449-56. [Crossref] [PubMed]
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, Predictors, and Outcomes of Aortic Regurgitation After Transcatheter Aortic Valve Replacement: Meta-Analysis and Systematic Review of Literature. J Am Coll Cardiol 2013;61:1585-95. [Crossref] [PubMed]
- Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation following transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation 2014;129:1233-43. [Crossref] [PubMed]
- Nazif TM, Dizon JM, Hahn RT, et al. Predictors and Clinical Outcomes of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) Trial and Registry. JACC Cardiovasc Interv 2015;8:60-9. [Crossref] [PubMed]
- Dizon JM, Nazif TM, Hess PL, et al. Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart 2015;101:1665-71. [Crossref] [PubMed]


