Practical clinical applications of 3-D printing in cardiovascular surgery
The authors are an engineer and two surgeons who developed a shared interest in three-dimensional (3-D) printing of the ascending aorta in 2000, leading to the development of a customised external support for the Marfan aortic root (1). The invitation from the Journal of Thoracic Disease to write on the subject of 3-D printing provides an opportunity to briefly illustrate some existing, practical but very different applications. The fundamental attraction of 3-D printing over the usual range of surgical illustrations is that it provides a ‘graspable’ object (2). The word ‘graspable’ which we have borrowed from Regnier, is particularly appropriate in this context in that it indicates a physical object which the surgeon can literally grasp, manipulate in the hands (3,4), and cut in operative rehearsals (5). Being able to manipulate the object while simultaneously viewing it may have a significant impact on the understanding of 3-D anatomy by the surgeon.
We searched PubMed for 2017 up to mid-July using the search terms [3-D printing] OR [Three-dimensional printing] AND [Surgery]. Of a total of 40 papers returned, 28 related to orthopaedic, plastic and reconstructive surgery. Only six related to 3-D printing in cardiovascular surgery, usually at a case report level (5-9). The relative sparsity of clinical publications indicates the innovative nature of the report by Chen et al. (3). In addition the search returned several excellent technical explanations dealing with image acquisition, data manipulation and the technology, and the clinical application involved in 3-D printing (10-13). In this article, using the verb ‘to grasp’ in its figurative sense, our purpose is to make the concepts graspable by clinical surgeons.
Terminology: from rapid prototyping (RP) to 3-D printing
In 2000, the patient/engineer (TG) introduced the surgeon/researcher (TT) to RP during a meeting of the Marfan Association, a British patients’ group (1). At the time the technique, referred to as ‘RP’, was familiar to maxillo-facial surgeons (14,15) but generally not to cardiovascular surgeons. Prototyping in industry, which is where the term comes from, means creating a first sample of something that in due course might become a mass produced manufactured item (15). What is meant now, in the context we will describe, is a unique product, customised for an individual patient for a specific purpose. With respect to the manufacturing process ‘additive manufacture’ (5) is a catch-all term for selective laser sintering, stereolithography (6,16), fused deposition modelling, and various other emerging techniques (15). These make the process distinct from ‘subtractive manufacturing’ which starts with a block of a material which is machined away to form the finished item, effectively like hand carving a stone sculpture. Given the mathematical processing and the manufacturing time required, ‘rapid’ in the sense understood by surgeons is not achievable so neither ‘rapid’ nor ‘prototype’ fit the context. We will therefore use the current popular term, 3-D printing.
Computer-aided design or ‘CAD’ modelling
Three clinical narratives
3-D printing to grasp complex anatomy: a right-sided aortic arch and associated pathology
A right sided arch maybe associated with variable and unfamiliar anatomy. It may remain functional, asymptomatic, and undiscovered throughout life. If symptoms occur, 2-D imaging may lead to the discovery of a life-threatening or severely symptomatic situation which may be resolved by surgery as in the case described by Chen et al. (3) or another we will introduce here (Figure 1) in which a Type B dissection occurred in an undiagnosed right sided arch (17).
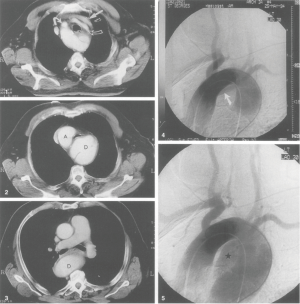
In 1994, a man of 72 presented with sudden onset severe chest pain at a district hospital. A diagnosis of Type B dissection was made. He was hypertensive. The management plan was appropriate to the clinical situation and he was managed conservatively. After three months he presented again with stridor, breathlessness and a bovine cough. A chest radiograph showed enlargement of the aorta and he was admitted and transferred as an emergency to the cardiothoracic surgical unit. No mention had been made of the side of the aortic arch: in the conservative management of a 72-year-old hypertensive it was not of immediate relevance. On the evening of the emergency admission preparations were made for surgery. The radiologist Dr. Alan Wilson (18) appeared at the operating room door with CT films in his hand. Perhaps feigning curiosity, he tactfully asked the surgeon (TT) if there were any special considerations in operating on a right sided arch. After more than twenty years it is unsure whether the right sided arch had ever been suspected before being diagnosed by AW but what is certain is this: never has a surgeon been more grateful. Instead of being caught out, on the wrong side of the chest, the resection and replacement on the descending aorta with bypass support to the lower body were never so easily achieved. In the event the right sided arch was diagnosed on 2-D imaging and the timely intervention of the radiologist averted a disaster. In the presence of aortic dissection and dilatation, the side of the aortic arch and descending aorta had not been grasped by the clinicians.
As Sundararaghavan wrote in his editorial ‘more information means better outcomes’ (13). In Chen et al.’s elective operation, 3-D printing assisted operative planning in way not imagined by us in1994 (3).
Rehearsing an operation requiring precision resection
Hermsen and colleagues have recently described the use of 3-D printing to plan and practice septal myectomy in two patients with hypertrophic cardiomyopathy (5). The exact location and depth of resection is critical: not too much but not too little either (Figure 2). We introduce a historical note, again to illustrate the advance that 3-D printing represents.
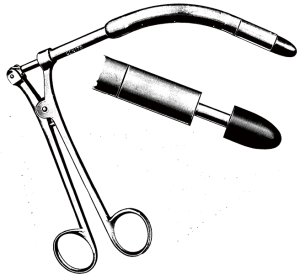
In 1947 Alfred Blalock of John’s Hopkins went to Guy’s Hospital, London and taught Brock the subclavian to pulmonary artery shunt operation to palliate cyanotic heart disease associated with obstruction to pulmonary blood flow (19,20). Blalock had respected the dictum ‘Noli tangere: do not touch. Do not touch the heart’ (21). Brock wanted to move on to ‘direct’ operations to not just bypass the obstruction, but to relieve both the valvar and subvalvar obstruction (19,22) (Figure 3). In 1948 Brock introduced Blalock to his operation (right ventricular myectomy). Blalock wrote: ‘The operation is that which has been described by Mr. Brock of Guy’s Hospital, London, and in fact he performed the operation on seven of the patients described in this report while he was serving as an Exchange Professor in The Johns Hopkins Medical School and Hospital’ (23). In 1949 Brock did his operation on the beating heart using a punch introduced through the anterior surface of the right ventricle. He was unable to see the anatomy; it was all done by feel (Figure 4).
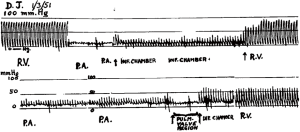
Pre-operative imaging was limited to angiocardiography producing still radiographs at two frames per second with a Fairchild camera (19). However, Brock had the advantage that he could monitor the progress of the relief of the obstruction by direct pressure measurements in the right ventricle and the pulmonary artery and desist when the pressures difference was judged to be acceptable, and there was a good pulse and pressure wave in the pulmonary artery. (Figure 5) It raises the point that left ventricular myectomy may be better done as an image guided intravascular intervention on a beating heart with monitoring of pressures either side of the obstruction. Nevertheless 3-D printing might be a valuable adjunct.
Individualised device manufacture
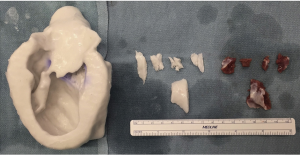
In our own current clinical application, digital imaging data are used to make a 3-D copy of the individual’s aorta and on that is manufactured a personalised external aortic root support (PEARS) made of a mesh fabric of the same tried and tested polymer (polyethylene terephthalate) as has been used for half a century in vascular grafts (4). This has now been done in over 100 patients. The mesh has intrinsic strength preventing any subsequent enlargement of the aortic root dimensions. We also know from detailed animal experiments (25), corroborated with the histology from human aorta/mesh composite after 4–5 years, that the mesh acts as a scaffold and its 0.7 mm pore size allows the mesh to be fully incorporated in the aortic wall, while the valve support and the blood/endothelial interface remains completely undisturbed. PEARS uses the 3-D model of a patient’s aorta, not as a replacement itself, but as a step on the way to the manufacture of a customised implant (Figure 6).

Conclusions
The digital imaging data may be derived from computerized tomography (3,4) or magnetic resonance images (4) or any other process from which information can be exported as voxel-based tomographic images. This is the limiting step in the precision of the model. The potential resolution of the manufacturing processes at present is generally significantly higher than the CT image/voxel resolution. The various cited authors provide information about the files exported from the imaging software programmes used for CAD and the process used to create the model (3). What becomes clear is that there are many ways of going about these steps but that in principle, 3-D printing is a technology with wide and varied applications, each of which will have to be carefully considered and evaluated.
Acknowledgements
None.
Footnote
Conflicts of Interest: TG is the inventor of the PEARS device and a shareholder in Exstent Ltd. The other authors have no conflicts of interest to declare.
References
- Golesworthy T, Lampérth M, Mohiaddin R, et al. The Tailor of Gloucester: a jacket for the Marfan's aorta. Lancet 2004;364:1582. [Crossref] [PubMed]
- Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010;5:335-41. [Crossref] [PubMed]
- Chen N, Zhu K, Zhang H, et al. Three-dimensional printing guided precise surgery for right-sided aortic arch associated with Kommerell's diverticulum. J Thorac Dis 2017;9:1639-43. [Crossref] [PubMed]
- Treasure T, Petrou M, Rosendahl U, et al. Personalized external aortic root support: a review of the current status. Eur J Cardiothorac Surg 2016;50:400-4. [Crossref] [PubMed]
- Hermsen JL, Burke TM, Seslar SP, et al. Scan, plan, print, practice, perform: Development and use of a patient-specific 3-dimensional printed model in adult cardiac surgery. J Thorac Cardiovasc Surg 2017;153:132-40. [Crossref] [PubMed]
- Fujita T, Saito N, Minakata K, et al. Transfemoral transcatheter aortic valve implantation in the presence of a mechanical mitral valve prosthesis using a dedicated TAVI guidewire: utility of a patient-specific three-dimensional heart model. Cardiovasc Interv Ther 2017;32:308-11. [Crossref] [PubMed]
- Jaworski R, Haponiuk I, Chojnicki M, et al. Three-dimensional printing technology supports surgery planning in patients with complex congenital heart defects. Kardiol Pol 2017;75:185. [Crossref] [PubMed]
- Kappanayil M, Koneti NR, Kannan RR, et al. Three-dimensional-printed cardiac prototypes aid surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases: Early experience and proof of concept in a resource-limited environment. Ann Pediatr Cardiol 2017;10:117-25. [Crossref] [PubMed]
- McGovern E, Kelleher E, Snow A, et al. Clinical application of three-dimensional printing to the management of complex univentricular hearts with abnormal systemic or pulmonary venous drainage. Cardiol Young 2017;27:1248-56. [Crossref] [PubMed]
- Ripley B, Levin D, Kelil T, et al. 3D printing from MRI Data: Harnessing strengths and minimizing weaknesses. J Magn Reson Imaging 2017;45:635-45. [Crossref] [PubMed]
- Li C, Cheung TF, Fan VC, et al. Applications of Three-Dimensional Printing in Surgery. Surg Innov 2017;24:82-8. [Crossref] [PubMed]
- Bartel T, Rivard A, Jimenez A, et al. Medical three-dimensional printing opens up new opportunities in cardiology and cardiac surgery. Eur Heart J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Sundararaghavan S. Three-dimensional-printed cardiac prototypes in complex congenital cardiac defects: New technology with exciting possibilities. Ann Pediatr Cardiol 2017;10:114-6. [Crossref] [PubMed]
- McGurk M, Amis AA, Potamianos P, et al. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl 1997;79:169-74. [PubMed]
- Wong K, Hernandez A. A review of additive manufacturing. ISRN Mechanical Engineering 2012;2012:1-10. [Crossref]
- Sodian R, Weber S, Markert M, et al. Stereolithographic models for surgical planning in congenital heart surgery. Ann Thorac Surg 2007;83:1854-7. [Crossref] [PubMed]
- Senthil V, Treasure T. Type B dissection involving a right-sided aortic arch. Eur J Cardiothorac Surg 1996;10:477-9. [Crossref] [PubMed]
- Marchbank N, Wilson N, Jones JG, et al. Munk’s Roll Lives of the Fellows: Alan George Wilson 1936-2012. Available online: http://munksroll.rcplondon.ac.uk/Biography/Details/6555. Accessed 29/7/2017.
- Treasure T. The Heart Club. ed 1st, London/New York, Clink Street, 2017.
- Campbell M, Deuchar D. Results of the Blalock-Taussig operation in 200 cases of morbus caeruleus. Br Med J 1953;1:349-58. [Crossref] [PubMed]
- Silverman P, Caswell R. 'Something the Lord Made' 2004 Biographical movie about Vivien Thomas. Available online: http://www.script-o-rama.com/movie_scripts/s/something-the-lord-made-script.html. Accessed 29/7/2017.
- Brock RC, Campbell M. Infundibular resection or dilatation for infundibular stenosis. Br Heart J 1950;12:403-24. [Crossref] [PubMed]
- Blalock A. Surgical procedures employed and anatomical variations encountered in the treatment of congenital pulmonic stenosis. Surg Gynecol Obstet 1948;87:385-409. [PubMed]
- Brock RC. Direct cardiac surgery in the treatment of congenital pulmonary stenosis. Ann Surg 1952;136:63-72. [Crossref] [PubMed]
- Van Hoof L, Verbrugghe P, Verbeken E, et al. Support of the aortic wall: a histological study in sheep comparing a macroporous mesh with low-porosity vascular graft of the same polyethylene terephthalate material. Interact Cardiovasc Thorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]