A comparative study of conventional cytology and cell block method in the diagnosis of pleural effusion
Introduction
Thoracentesis is a diagnostic procedure for patients with pleural effusion. Pleural fluid (PF) obtained from the procedure should be submitted for biochemical, microbiological, and cytological study (CS). In cases of suspicion of malignant pleural effusion (MPE), CS is extremely useful as it provides a diagnostic rate of 60%, ranging from 40% to 87% (1-4). CS is important not only in diagnosis but also in staging and further guiding treatment for malignancy. Many widely used guidelines, such as those of the American College of Chest Physicians (ACCP) and the British Thoracic Society (BTS), recommend CS of two samples of pleural effusions (1,4). If the procedures turn out to be non-diagnostic, further invasive investigations such as imaged-guided pleural biopsy or thoracoscopic biopsy are recommended for a definitive diagnosis.
The challenges of obtaining a diagnosis from CS include indistinct morphological details, overlapping or overcrowding of cells, abundance of inflammatory cells, paucity of representative cells, and cell losses or changes (5). To overcome these limitations, cell block (CB) method was developed to provide better tissue architecture and morphological features for differentiating between malignant and non-malignant cells and also for further processing via special stains and immunohistochemistry (6). Although CB technique has been known for nearly a century, there have been few reports and a limited number of samples involving the direct comparison of CS and CB on consecutive patients for diagnosis of pleural effusion (5,7-12). In addition, most studies have focused on the diagnostic yield in MPE. The aim of this study was to compare the diagnostic yields of CS, CB technique and the combination of both, regardless of the etiology of pleural effusion.
Methods
Patients
A cross-sectional study was conducted at Ramathibodi Hospital, Mahidol University, Thailand, from June 2015 to May 2016 on patients >15 years of age with pleural effusion, as demonstrated on chest radiographs, who underwent thoracentesis for diagnostic purposes. Written informed consent was obtained from all patients before commencement of the procedure. The study protocol was approved by the Ethics Committee on Human Experimentation of Ramathibodi Hospital, Faculty of Medicine, Mahidol University (ID 05-58-15).
PF was sent for biochemical [protein, glucose, lactate dehydrogenase, and adenosine deaminase (ADA) level] and microbiological [Gram and acid-fast bacilli (AFB) staining and bacterial and mycobacterial culture] analysis, white blood cell count and differential count, and CS and CB for diagnostic evaluation. Other diagnostic investigations of PF were performed according to clinical suspicions.
CS
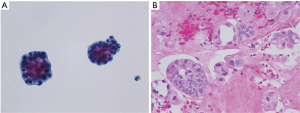
15 mL of fresh PF was centrifuged at 2,500 rpm for 20 min and the supernatant removed. One direct slide smear was prepared from cell sediment and submitted for Papanicolaou staining. 15 mL of CytoLyt® solution (Hologic, Marlborough, MA, USA) was added to the remaining sediment and centrifuged at 600 rpm for 20 min. The supernatant was poured off and the sediment was placed into a vial of PreservCyt® solution (Hologic) and left to stand for 15 min. The vial was run on an automated ThinPrep® 2000 processor (Hologic) giving one liquid-based slide. The slide was fixed and submitted for Papanicolaou staining. Both conventional and liquid-based slides were sent for cytological evaluation (Figure 1A).
CB study
15 mL of fresh PF was centrifuged at 6,000 rpm for 5 min and the supernatant removed. Agar solution was added to the specimen, followed by refrigeration to form a solid clot. The clot was fixed in 10% neutral buffered formalin solution and automatically processed into a paraffin-embedded block. A histological slide was cut and hematoxylin and eosin (H&E) staining was performed (Figure 1B). Microbiological and immunohistochemical (IHC) stains were applied in cases where special staining was indicated and requested.
A diagnosis was established when CB or CS results were defined as malignant disease or specific non-neoplastic disease. CB or CS diagnosis of non-specific inflammation was considered to be non-diagnostic, although the final diagnosis proved to be a benign process. The final diagnosis in non-diagnostic CB or CS results was reached by thoracoscopic pleural biopsy, biochemical results, microbiological results, clinical manifestations, radiographic findings, and improvement or progression on treatment and follow-up.
Statistical analysis
All data were analyzed with a statistical software package (SPSS for Windows version 16.0; SPSS, Chicago, IL, USA). Values were expressed as mean ± standard deviation for continuous variables, and as frequencies and percentages for categorical variables. The diagnostic yields of CS and CB were compared using McNemar’s test. All statistical tests were two-sided and P<0.05 was considered to be statistically significant.
Results
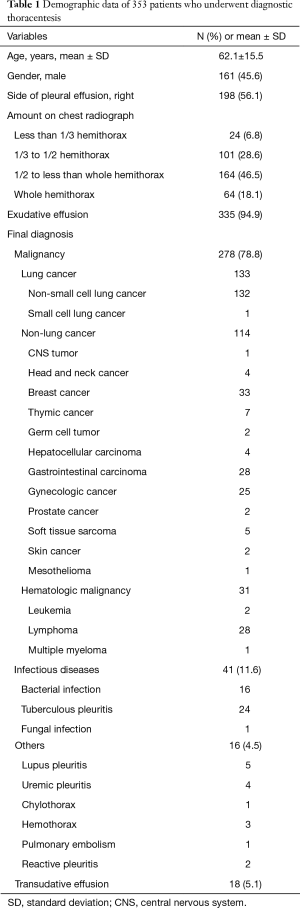
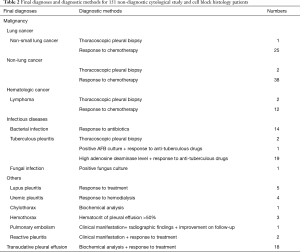
There were 353 patients, comprising 161 males and 192 females with a mean age of 62.1±15.5 years. The demographics of the study population are shown in Table 1. CS and CB provided similar diagnostic yield (48.7% and 49.9%, respectively; P=0.69). However, a combination of CS and CB gave a higher yield (57.2%) than CS alone (P<0.001). The diagnostic methods for the 151 patients with non-diagnostic CS or CB are shown in Table 2. For cancer patients for whom definite etiologies of pleural effusions were not achieved by cytopathology, primary cancers were all established and pleural effusions decreased after chemotherapy.
Full table
Full table
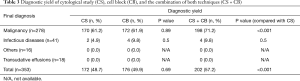
Regarding the etiologies of pleural diseases, CS and CB rendered similar diagnostic yields in MPE (61.2% and 61.9%, respectively; P=0.89). In 108 negative CS MPE patients, CB revealed malignant disease in 28 patients (25.9%) and a combination of CS and CB improved the diagnostic yield significantly compared with CS (P<0.001) (Table 3). In infectious pleuritis, CS could diagnose in one case of empyema thoracis and one tuberculous pleuritis. CB provided additional diagnosis in one other case of empyema thoracis and one tuberculous pleuritis. In other inflammatory diseases and transudative pleural effusions, CS and CB had no role in diagnosis.
Full table
CB achieved a diagnostic yield similar to that of CS in all subgroup of malignancies. Although not reaching statistical significance, CB tended to have higher diagnostic yield than CS in hematologic malignancy (Table 4). Submission of CB improved the diagnostic yield to CS in all subgroup of malignancies.
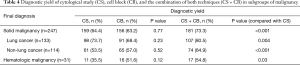
Full table
All CB specimens with confirmation of malignancy that underwent more specific evaluations as requested by the oncologists were adequate for further examination, including IHC staining in 87 patients (lung cancer, 38; non-lung cancer, 37; and hematologic malignancy, 12) and molecular testing for lung cancer [epidermal growth factor receptor (EGFR) mutations, 63; and anaplastic large-cell lymphoma kinase (ALK) rearrangements, 39].
Discussion
Diagnosis of pleural disease can be made by direct examination of the pleura itself or by indirect evaluation of PF that accumulates in the pleural cavity. As it is a non-invasive technique and available in most hospitals, thoracentesis to retrieve PF for examination is well accepted as an initial investigation. In MPE, CS from PF provides a diagnostic rate of 60%, ranging from 40% to 87% (1-4). During thoracentesis, closed-blind pleural biopsy can be performed simultaneously to obtain pleural tissue for histology. However, its diagnostic yield is less sensitive than CS, as pleural metastases tend to be focal in the parietal pleura (13,14). In addition, pleural biopsy sometimes fails to provide adequate tissue. Furthermore, complications such as pneumothorax, hemothorax, extravasation of PF, and injury to adjacent organs may occur. Therefore, closed-blind pleural biopsy is not routinely recommended in all cases, and has been replaced by image-guided pleural biopsy.
To enhance the diagnosis from cytohistology of PF, the sediment from centrifuged PF can be processed as CB for histology. Although CB technique has been described for nearly a century and is well-known among cytopathologists, it is still underprescribed by clinicians. Most of the published studies were conducted by cytopathologists, and PF samples selected for investigation had already been submitted to the laboratory, conditions which do not resemble daily clinical practice. Therefore, we conducted this study to explore the benefits of CB when integrated as a part of PF examination in routine clinical practice.
There are many fixative substances for binding the sediment cells before embedding into paraffin blocks, including formalin, alcohol-formalin, alcohol-acetic acid-formalin, agar, plasma thrombin clot, CytoLyt-prefixed thrombin clot, HistoGelTM, and inverted filter sedimentation (5-8,15). Although some studies have evaluated cell morphology and IHC performance of CB using different fixatives (6,15), there is no consensus guideline in this process, leaving the choice up to each institute based on availability and cost-affordability. In this study, agar solution was used to form a solid clot. The cost per CB sample was $27 US.
In MPE diagnosis, CB has certain advantages over CS. Improper smear, fixation, and staining techniques in CS can cause cell overlapping or overcrowding, cell loss, artifacts, and poor background staining, while these are less frequent in CB (5-8). Cellularity is higher by CB compared with CS and is concentrated in one small area that can be evaluated at a glance, with all cells lying in the same focal plane of the microscope (11,12). In addition, CB provides better cellular morphological details, such as better nuclear and cytoplasmic preservation, intact cell membrane and crisp chromatin; there is also less difficulty in microscopic observation, in spite of the presence of excess blood in the background (12). Regarding tissue architecture, adenocarcinoma cells, especially from the lung, breast, and gastrointestinal tract, may not clearly exhibit cellular morphology of malignancy; better morphological details and tissue architecture pattern are required for diagnosis (5,11,12). Unfortunately, CS has the limitation of a lack of tissue architecture. Singly scattered cells are predominantly found in CS, whereas architectural patterns such as glands, sheets, three-dimensional cell clusters, and cell balls are commonly demonstrated in CB, resulting in increased sensitivity of diagnosis of MPE by CB method (12). Discrimination of reactive mesothelial cells and malignant cells is a major challenge in CS, as reactive mesothelial cells may express large irregular nucleoli, coarse chromatin, and enlarged nuclei, mimicking malignancy (5,7,8,11,12). In contrast, in CB the nucleoli are not as prominent as in CS and the pseudo-acinar or acinar structures can be better appreciated (11,12). Finally, CB specimens can be stored and multiple sections performed by routine staining, special staining, IHC staining, and also molecular testing as in our study and previous reports (16,17), while storage of CS remains a practical problem. However, CB entails a risk of losing material during preparation, especially in the case of fixation technique (18) that might explain negative CB results but positive CS results in some cases. Similar to our results, previous studies showed an additional diagnostic rate of CB to CS around 10–15% in MPE (5,7,8,12,19).
In tuberculous pleuritis, granulomatous inflammation, which is a key to cytopathological diagnosis, usually occurs throughout the parietal pleura, resulting in a high diagnostic yield of closed-blind pleural biopsy (2,4,20,21). In contrast, this reaction rarely exfoliates into the PF, and consequently there are low diagnostic yields of CS and CB. PF-ADA level of greater than 40 U/L achieves a high diagnostic rate similar to that of closed-blind pleural biopsy, which may render routine closed-blind pleural biopsy unnecessary in the initial thoracentesis (21).
As expected, CS and CB had no role in diagnosis of non-infectious inflammatory pleuritis and transudative effusion. Clinical manifestations and biochemical analysis are required to obtain a diagnosis in these conditions.
There were some limitations in our study. As pleural effusion in cancer patients does not usually resemble MPE (so-called paramalignant effusion), a clinical diagnosis of response to chemotherapy may not be valid for concluding a final diagnosis of MPE in negative CS and CB patients. We did not perform other invasive diagnostic procedures to reach a final diagnosis when there was no any clinical benefit to the patients as suggestion by the oncologists, especially in known advanced malignant diseases. In addition, we did not routinely perform special staining to define all subtypes of malignancies if there was no treatment benefit. However, if required, all CB specimens with confirmation of malignancy were adequate for further examination.
In conclusion, we demonstrated that in MPE, CB method provided a similar diagnostic performance to CS, while submission of both techniques can significantly increase the diagnostic yield. However, in other pleural diseases, CB and CS had limited values in diagnosis, requiring clinical presentation as well as biochemical and microbiological examinations of PF.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study protocol was approved by the Ethics Committee on Human Experimentation of Ramathibodi Hospital, Faculty of Medicine, Mahidol University (ID 05-58-15).
References
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- McGrath EE, Anderson PB. Diagnosis of pleural effusion: a systematic approach. Am J Crit Care 2011;20:119-27. [Crossref] [PubMed]
- Gupta S, Sodhani P, Jain S. Cytomorphological profile of neoplastic effusions: an audit of 10 years with emphasis on uncommonly encountered malignancies. J Cancer Res Ther 2012;8:602-9. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii4-17. [Crossref] [PubMed]
- Köksal D, Demırağ F, Bayız H, et al. The cell block method increases the diagnostic yield in exudative pleural effusions accompanying lung cancer. Turk Patoloji Derg 2013;29:165-70. [Crossref] [PubMed]
- Jing X, Li QK, Bedrossian U, et al. Morphologic and immunocytochemical performance of effusion cell blocks prepared using 3 different methods. Am J Clin Pathol 2013;139:177-82. [Crossref] [PubMed]
- Ugurluoglu C, Kurtipek E, Unlu Y, et al. Importance of the cell block technique in diagnosing patients with non-small cell carcinoma accompanied by pleural effusion. Asian Pac J Cancer Prev 2015;16:3057-60. [Crossref] [PubMed]
- Bhanvadia VM, Santwani PM, Vachhani JH. Analysis of diagnostic value of cytological smear method versus cell block method in body fluid cytology: study of 150 cases. Ethiop J Health Sci 2014;24:125-31. [Crossref] [PubMed]
- Ghosh I, Dey SK, Das A, et al. Cell block cytology in pleural effusion. J Indian Med Assoc 2012;110:390-2, 396. [PubMed]
- Shobha SN, Kodandaswamy CR. Utility of modified cell block technique in cases of pleural effusion suspected of malignancy. Int J Health Sci Res 2013;3:33-8.
- Dekker A, Bupp PA. Cytology of serous effusions. An investigation into the usefulness of cell blocks versus smears. Am J Clin Pathol 1978;70:855-60. [Crossref] [PubMed]
- Shivakumarswamy U, Arakeri SU, Karigowdar MH, et al. Diagnostic utility of the cell block method versus the conventional smear study in pleural fluid cytology. J Cytol 2012;29:11-5. [Crossref] [PubMed]
- Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985;60:158-64. [Crossref] [PubMed]
- Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol 1991;4:320-4. [PubMed]
- Nigro K, Tynski Z, Wasman J, et al. Comparison of cell block preparation methods for nongynecologic ThinPrep specimens. Diagn Cytopathol 2007;35:640-3. [Crossref] [PubMed]
- Liu X, Lu Y, Zhu G, et al. The diagnostic accuracy of pleural effusion and plasma samples versus tumour tissue for detection of EGFR mutation in patients with advanced non-small cell lung cancer: comparison of methodologies. J Clin Pathol 2013;66:1065-9. [Crossref] [PubMed]
- Wang W, Tang Y, Li J, et al. Detection of ALK rearrangements in malignant pleural effusion cell blocks from patients with advanced non-small cell lung cancer: a comparison of Ventana immunohistochemistry and fluorescence in situ hybridization. Cancer Cytopathol 2015;123:117-22. [Crossref] [PubMed]
- Kung IT, Yuen RW, Chan JK. Optimal formalin fixation and processing schedule of cell blocks from fine needle aspirates. Pathology 1989;21:143-5. [Crossref] [PubMed]
- Thapar M, Mishra RK, Sharma A, et al. Critical analysis of cell block versus smear examination in effusions. J Cytol 2009;26:60-4. [Crossref] [PubMed]
- Ruan SY, Chuang YC, Wang JY, et al. Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 2012;67:822-7. [Crossref] [PubMed]
- Behrsin RF, Junior CT, Cardoso GP, et al. Combined evaluation of adenosine deaminase level and histopathological findings from pleural biopsy with Cope's needle for the diagnosis of tuberculous pleurisy. Int J Clin Exp Pathol 2015;8:7239-46. [PubMed]


