Pleurodesis: a comparison of two sclerosing agents for pleural effusions in Mozambique
Introduction
Pleural effusions constitute one of the most frequent pathologies encountered in hospitalized patients in the pulmonary service of Maputo Central Hospital (MCH) in Mozambique. Most effusions are infectious in origin and require drainage with treatment of the underlying cause of the effusion. In 2010, 150 (50%) of the 302 thoracenteses performed in the pulmonary medicine ward revealed results consistent with Kaposi’s sarcoma (unpublished data). Previous studies have demonstrated that amongst malignant pleural effusions, lung cancer, metastatic breast cancer, lymphomas and Kaposi’s sarcoma are responsible for 75% of these effusions (1). The majority of these recur after simple thoracentesis within 5–10 days. Repeated thoracenteses in these cases are not recommended as this process can increase the risk of metastatic spread at the site of the puncture, pneumothorax, empyema, and loss of protein (2,3). The resulting protein depletion leads to a decrease in oncotic pressure and consequent new accumulation of fluid in the pleural space (2). In this situation, patients present with shortness of breath that interferes with their quality of life. Placement of a pleural drainage tube and thoracoscopy with installation of sclerosing agents in the pleural space is the preferred approach for these recurrent effusions (4-6). Indwelling catheters have not been found to be more effective than talc pleurodesis with chest tube drainage and in those with prolonged survival pleurodesis appears to be the most cost effective treatment (3,6).
Pleurodesis aims to provoke an inflammatory process within the visceral and parietal pleura that will fibrose and adhere both pleural layers together thereby preventing reaccumulation of fluid (1,7). The mechanism of pleurodesis at the cellular and molecular level involves substantial and widespread irritation of the mesothelium that causes recruitment of fibroblasts with subsequent deposition of collagen in the pleural space and activation of the coagulation cascade and inhibition of fibrinolytic activity in the pleural space (1).
At MCH we currently lack an optimal sclerosing agent based on financial constraints and lack of efficacy data. On the basis of this conceptual framework, we aimed to retrospectively compare the efficacy and safety of sodium hydroxide (NaOH) with bleomycin for pleurodesis and observe the side effects triggered by these agents. Concurrent with this goal, we estimated the cost of pleurodesis associated with these agents.
Methods
Participants, setting and data collection
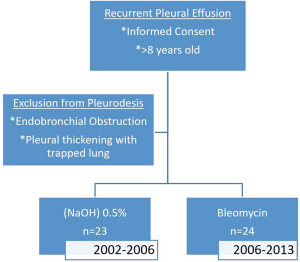
We conducted a retrospective analytic cohort study of patients who underwent pleurodesis for recurrent pleural effusions using bleomycin or NaOH from 2002 to 2013. Standard of care for pleurodesis in cases of recurrent pleural effusions at MCH was developed using the materials available. NaOH remained the agent of choice until 2006 when bleomycin became available in the oncology unit. Ethics approval for use of patient’s records was obtained from the institutional review board of MCH and Faculdade de Medicina Eduardo Mondlane (Combined IRB of Eduardo Mondlane University: CIBSFM HCM/39/2014). Patient confidentiality was maintained by assignment of a unique, encrypted identifier for each record. Clinical data regarding general complications, rate of success and lung expansion were noted for every patient who underwent pleurodesis at MCH during this time frame.
Inclusion criteria
We included patients at least 8 years of age with recurrent pleural effusion (malignant or benign) of moderate to large volume who provided informed consent for pleurodesis. The patients all had respiratory symptoms relieved after thoracentesis and achieved 100% lung expansion after thoracentesis. All participants had a life expectancy >3 months and a reasonable performance status (Karnofsky >70).
Exclusion criteria
The exclusion criteria included inability to provide informed consent for the pleurodesis procedure, endobronchial obstruction and pleural thickening with trapped lung.
Pleurodesis technique (2,8)
Posteroanterior and lateral chest X-ray were performed prior to introduction of the catheter. Tube thoracostomy was performed in the posterior axillary line in the 8–9th intercostal space with connection to a water sealed drainage system. Gentle aspiration occurred over 24 h or until the drainage stops or did not exceed 60–100 mL/24 h with subsequent lung expansion. Repeat chest X-ray assessed for removal of all the liquid and lung expansion. Sclerosing agent was injected through the tube followed by injection of 20 mL of normal saline after which the tube was clamped. The patient changed position during the following 4–6 h after which the tube was unclamped and attached to the drainage system with active drainage until the volume of liquid drained did not exceed 60 mL/24 h. If the repeat chest X-ray demonstrated lung re-expansion and absence of pleural effusion the drainage catheter was removed. If a pleural effusion was present, the drainage system was continued for an additional 24 h. Pleurodesis was repeated using the same sclerosing agent if after 48 h the drainage exceeded 200 mL. No intra-pleural anesthetics during pleurodesis or anti-inflammatory medications during or 1 week post procedure were used.
In cases where the initial pleurodesis for malignant pleural effusions failed, the following alternatives were considered: repeat therapeutic thoracentesis, pleurectomy, or repeat pleurodesis several months following initial pleurodesis (9).
Definitions
We defined clinical success rate as an effective expansion of the lung to the chest wall (100% efficiency) confirmed by physical exam, radiographic resolution combined with numbers of pleurodeses required to achieve symptomatic relief (9). Partial success rate was defined by clinical signs and chest X-ray that indicates the presence of residual pleural fluid, <50% of the initial radiograph (in this case the efficiency is 60–70%). Recurrent effusions are defined by reappearance of pleural fluid within 1 week after first thoracentesis.
Statistical analysis
Frequencies and proportions for baseline characteristics and clinical outcomes were reported. Frequencies of clinical outcomes and complications for each treatment group were compared using Fisher’s exact test and P values are reported. Significance testing was done using a two-tailed alpha level of 0.05. All analysis was done using SAS® Studio statistical software, version 3.6.
Results
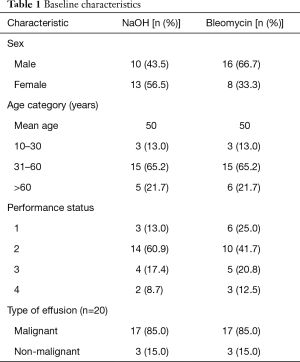
A total of 47 patient case records of pleurodesis were identified, of which 24 cases used bleomycin and 23 cases used NaOH as the sclerosing agent (Figure 1). Patient characteristics were balanced between the 2 groups and majority of pleural effusions were malignant in origin (Table 1). As a retrospective study, various components of the patient record were incomplete, however, the data were analyzed with inclusion of all 47 patient case records.
Full table
Complications noted for each of these procedures included: general complications (fever, nausea, cough and shortness of breath) and local pain (Table 2). There was a statistically significant difference in the incidence of fever between the 2 groups (0% in the NaOH group vs. 23.8% in the bleomycin group, P=0.048).

Full table
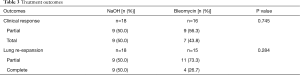
There was no statistically significant difference between the use of bleomycin and NaOH in partial or complete clinical success and lung expansion by radiographic (Table 3). Only three patients had to undergo repeat pleurodesis for recurrent symptomatic malignant pleural effusions, all of which underwent initial pleurodesis with NaOH. However, no specific characteristics of these patients appeared to be predictive of failure of the first pleurodesis.
Full table
We then analyzed the associated cost of each sclerosing agent. NaOH materials cost $0.78/bottle (0.5% NaOH prepared in 20 mL bottle) with 1 bottle used per treatment. In contrast, for bleomycin 1 bottle (5 mL=15 units) 1 U/kg or 1 mg/kg [e.g., 50–70 kg patients use 4 bottles (60 U)]. $40/bottle with average cost $160 per treatment.
Discussion
The search for a low-cost, effective and safe agent for treatment of recurrent pleural effusions has become important at MCH given the high prevalence of recurrent pleural effusions. This study has demonstrated that bleomycin and NaOH did not differ in outcomes or specific complication rate, though there was a statistically significant decrease in general complications and a trend towards improved rate of success and total lung expansion with NaOH. Given the financial pressures within the public health system in Mozambique, NaOH represents a cheaper and potentially more effective agent for pleurodesis as compared to bleomycin.
There are a wide variety of sclerosing agents available which include talc, antibiotics (tetracycline), cytostatic, antimalarials (quinacrine, mepacrine), 50% glucose in water, immunomodulators, caustic substances, nitrates and even biological agents (1). Talc is the most widely used agent though there is a search for more effective agents underway (3,10). In practice, we have yet to find the ideal product in terms of cost and effectiveness at MCH. The 40-fold increased costs in bleomycin compared to NaOH without apparent differences in efficacy and complication rate make NaOH an attractive alternative sclerosing agent. MCH does not have any of the aforementioned products because of the prohibitive cost of these agents. NaOH is an irritant that had been first used in the 1950s then abandoned in favor of newer agents considered more effective though without rigorous study. It is not clear why this product is no longer used. To our knowledge, the first reference to clinical use of NaOH occurred in 1976 when Bezanilla injected 20 mL of 15% NaOH into 15 patients with pleural effusions secondary to breast cancer and achieved control in 14 of these patients, a 93% efficacy rate (11). The study referred to side effects of pain that resolved rapidly and discharge from the hospital by the 3rd day after pleurodesis. Other published studies have managed to obtain similar efficacy. It has been found that NaOH is a low-cost agent with easy manipulation and sterilization, does not cause intense pain, and carries minimal morbidity, and we have shown here an efficacy of 80–95%. All of these characteristics suggest that NaOH can be an effective sclerosing agent for pleurodesis (12). In order to perform pleurodesis successfully it is necessary to consider the following factors of poor prognosis: a pleural fluid (pH <7.20, glucose <60 mg/dL), presence of lymphangitis or chylothorax or Karnofsky performance status <70 (12).
Despite our study’s strengths we acknowledge a number of limitations. First, our study was not randomized and thus there exists a possibility of selection bias or some other unexplained source of variance regarding how patients were allocated to each group. However, our use of bleomycin was based on its availability and thus we are not aware of any reason why selection bias may have occurred. Second, we are aware that our sample size is modest and thus would advocate for larger studies which may be helpful in defining the prevalence of rare complications. Third, some would argue that video-assisted thoracoscopic surgery (VATS) may be a preferable technique to provide pleurodesis as compared to tube thoracostomy at least for some patients. However, VATS is a more expensive technique and thus not ideal given our goal of defining an inexpensive strategy. Fourth, our study was a single center study in which the underlying pathology for malignant effusions may well be different from other centers. Thus, our findings are limited to the population studied. Despite these acknowledged limitations we believe that our new findings are a useful addition to the literature and may help guide subsequent research.
Conclusions
Management of recurrent pleural effusions remains a challenge, particularly in the developing world with a paucity of resources to provide symptomatic relief that is balanced against cost effectiveness. NaOH may be an alternative agent for these patients. Further work remains to be done to investigate the role of NaOH in resource limited settings.
Acknowledgements
Administrative support for this study was made possible by staff of the Pulmonary Wards (MCH) and Department of Medicine, Division of Infectious Diseases, University of California, San Diego (UCSD). The project described was supported by Grant Number R24TW008908 and R24TW008910 from the Fogarty International Center (Medical Education Program Initiative). This award is supported by funds provided to the NIH and HRSA under the “Tom Lantos and Henry Hyde United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Reauthorization Act of 2008”, Public Law 110–293, which is more commonly known as the US Presidents 12 Emergency Plan for AIDS Relief (PEPFAR). Co-funding is also provided by the Office of AIDS Research and the Common Fund of the NIHH Office of the Director. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Research involving human participants was approved by the Bioethics Committee of the Faculty of Medicine and Universidade of Eduardo Mondlane/Maputo Central Hospital (CIBSFM HCM/39/2014). As a retrospective case study of de-identified patient information, individual patient informed consent is not required. Verbal consent for pleurodesis was obtained at the time of the procedure with the risks and benefits discussed as well as alternative therapies.
References
- Bouros D, Froudarakis M, Siafakas NM. Pleurodesis: everything flows. Chest 2000;118:577-9. [Crossref] [PubMed]
- Light RW, Lee YC. Textbook of Pleural Diseases. 2nd Edition. Boca Raton: CRC Press, 2008.
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Vargas FS, Teixeira LR, Carmo AO, et al. Pleurodesis: future prospects. J Pneumol 2000;26:307-12. [Crossref]
- Antunes G, Neville E. Management of malignant pleural effusions. Thorax 2000;55:981-3. [Crossref] [PubMed]
- Karkhanis VS, Joshi JM. Pleural effusion: diagnosis, treatment, and management. Open Access Emerg Med 2012;4:31-52. [Crossref] [PubMed]
- Rodriguez-Panadero F, Antony VB. Pleurodesis: state of the art. Eur Respir J 1997;10:1648-54. [Crossref] [PubMed]
- Ibarra-Pérez C. Pleurodesis en derrame pleural maligno. Rev Inst Nal Enf Resp Mex 2005;18:123-31.
- Hirata T, Yonemori K, Hirakawa A, et al. Efficacy of pleurodesis for malignant pleural effusions in breast cancer patients. Eur Respir J 2011;38:1425-30. [Crossref] [PubMed]
- Puri V, Pyrdeck TL, Crabtree TD, et al. Treatment of malignant pleural effusion: a cost-effectiveness analysis. Ann Thorac Surg 2012;94:374-9; discussion 379-80. [Crossref] [PubMed]
- Bezanilla AR. Letter: Treatment for malignant pleural effusions. Chest 1976;70:408-9. [Crossref] [PubMed]
- Vaz MC, Marchi E, Vargas FS. Pleurodesis: technique and indications. J Bras Pneumol 2006;32:347-56. [Crossref] [PubMed]