Highlighted version successful resection of a tracheal metastasis of rectal cancer: a case report
Introduction
A trachea is rarely subject to the metastasis of various types of cancer, including colorectal cancer. The frequency of tracheal metastasis (TM) from non-pulmonary malignancy is reported to be less than 2% (1). Furthermore, there are very few reports about the TM and its optical management has yet to be established (2).
To our knowledge, colorectal cancer is the most frequent malignancy associated with TM among various types of malignancies; however, only ten patients with TM of colorectal cancer have been reported. Only three cases underwent surgical resection (3-5), and two patients were treated by laser therapy (3,6). These reports mainly focused on the diagnostic methods of TM and no reports have discussed the optical treatment options, including surgery, of TM of colorectal cancer. Here, we report a successful tracheal resection of TM of colorectal cancer and discuss the appropriate treatment for TM based on the pathological finding.
Case presentation
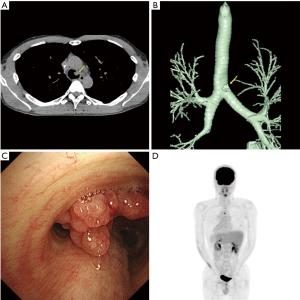
A 36-year-old man, who had been referred for blood in stool, underwent a surgical resection for the rectal cancer; and the pathological examination revealed the tumor as well to moderately differentiated adenocarcinoma. He received adjuvant chemotherapy with modified 5-fluorouracil and oxaliplatin 6. Computed tomography (CT) scan detected one lung metastasis in the right lower lung and one liver metastasis approximately 2 years after the surgery. Then, he received fluorouracil, leucovorin, and irinotecan (FOLFIRI) plus bevacizumab. Radiographic evaluation, performed every two cycles, revealed partial response in the lung and liver metastases. Then, partial resection of the lung and liver lesions was performed as a curative surgery. After the surgery, six cycles of FOLFIRI was administered. Although the patient has been free from new lesions for three years after the lung and liver surgery, carcinoid embryonic antigen level slightly increased. CT scan of the chest detected a lobulated intraluminal lesion arising from the left lateral tracheal wall (Figure 1A), and the volume-rendered image showed the lesion on 1.2 cm above the carina (Figure 1B). Bronchoscopic examination showed the lesion as a polypoid tracheal tumor (Figure 1C) and was pathologically diagnosed as a TM of rectal cancer. Fluorine-18 (F-18) fluorodeoxyglucose (FDG) positron emission tomography computed tomography (PET-CT) showed an uptake in TM [maximum standardized uptake value (SUV) =5.49] and without any significant intake of FDG in other organs (Figure 1D), and therefore, we subsequently underwent a tracheal resection and reconstruction as curative resection.
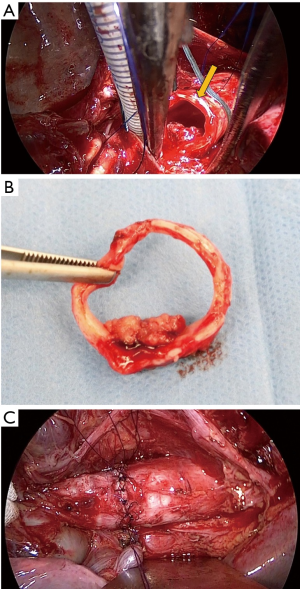
Through posterolateral incision, the chest cavity was opened through the 4th intercostal and left main bronchus and trachea was exposed. An incision on the lower trachea was made just beneath the carina and a ventilation tube (spiral tube 5.5 mm) was inserted to the left main bronchus. Then trachea was cut two rings away from the carinal cartridge, avoiding the incision to the tumor under bronchoscope (Figure 2A). One ring of trachea was removed with tumor (Figure 2B), and we confirmed surgical margin was negative by rapid intraoperative pathological diagnosis. JET-ventilation tube was inserted retrogradely and JET-ventilation was initiated. The end-to-end anastomosing suture was placed for the trachea reconstruction. Sutures were tied one by one after placing the suture 2 stitch forward. Finishing the ligation the anastomosing suture, water seal test was performed and no leak was found (Figure 2C).
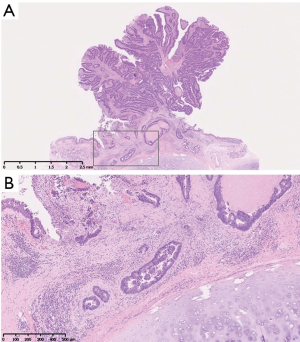
Pathological examination revealed the tumor as adenocarcinoma with tubular pattern, which was similar to primary rectal adenocarcinoma (Figure 3A). The carcinoma cells invaded the submucosal layer of tracheal wall (Figure 3B). The resected margins were free of carcinoma cells. Six months after the tracheal resection, he is doing well without any evidence of recurrence.
Discussion
The mechanism of TM has yet to be fully clarified; however, the mechanism of endobronchial metastasis is considered metastatic deposits, which are carried to the lung by pulmonary arteries or lymphatic channels, enter the peribronchial lymphatics and progress into the submucosal space (7). There exist several data which support this possible speculation: Kiryu et al. reported that 20 of 32 endobronchial lesions (62.5%) involved the submucosal layer (2); Taichman et al. also reported the invasion of the prominent angiolymphatics (8). In accordance with these reports, our pathological image also showed the involvement of the submucosal layer (Figure 3), with the possible angiolymphatic invasion of the tumor.
There are several treatment options for TM: surgery, bronchoscopic treatment, radiation therapy and chemotherapy. Bronchoscopic treatment is effective in reducing endotracheal obstruction or controlling hemoptysis for palliative treatment (9): there is a report which demonstrated the effectiveness of bronchoscopic electrocautery for removing a lesion (10). Moreover, another report showed the significance of laryngomicrosurgery for the TM (11). However, for curability of TM, the most optimal treatment of the TM would be a surgical resection, because TM often involves the submucosal layer or the angiolymphatics as mentioned above. Although snaring or argon plasma coagulation under bronchoscopy are often used for the TM with polypoid proliferation (12), there is a risk that the TM cannot be completely resected by such treatments, if there was an involvement of the submucosal layer. In fact, previous report showed that TM could not be completely extirpated by a laser microscope (6). Furthermore, Fournel et al. also reported that 13 among the 42 lesions (31%) were incompletely resected by bronchoscopic treatment (9). Given these reports and the current case, surgical resection of a trachea might be the curative treatment option, though such procedure is complicated, risky, and invasive with the need of much experience. The reported findings that most of the reported TM is a single lesion (10/11, 90.9%) may support the idea that surgical resection might be the curative therapy.
In conclusion, we suggest that the surgical resection of isolated TM for complete excision might be beneficial in increasing patients’ survival, although how long the surgical resection can prolong the prognosis should be clarified in the future study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Froudarakis ME, Bouros D, Siafakas NM. Endoluminal metastases of the tracheobronchial tree: is there any way out? Chest 2001;119:679-81. [Crossref] [PubMed]
- Kiryu T, Hoshi H, Matsui E, et al. Endotracheal/endobronchial metastases: clinicopathologic study with special reference to developmental modes. Chest 2001;119:768-75. [Crossref] [PubMed]
- Galbis Caravajal JM, Sales Badía JG, Trescolí Serrano C, et al. Endotracheal metastases from colon adenocarcinoma. Clin Transl Oncol 2008;10:676-8. [Crossref] [PubMed]
- Schmidt AH, Christensen TD, Madsen LB, et al. Endotracheal metastasis from colon cancer: A rare case. Open J Thorac Surg 2015;5:21-5. [Crossref]
- Watanabe S, Oda M, Ohta Y, et al. Endotracheal metastasis of rectal cancer. Eur J Cardiothorac Surg 2002;21:924. [Crossref] [PubMed]
- Choi IY, Lee KY, Lee JH, et al. Tracheal metastasis from rectal cancer: a case report and review of the literature. Balkan Med J 2013;30:120-2. [Crossref] [PubMed]
- Schoenbaum S, Viamonte M. Subepithelial endobronchial metastases. Radiology 1971;101:63-9. [Crossref] [PubMed]
- Taichman DB, Tino G, Aronchick J, et al. Diffuse airway narrowing from carcinoma metastatic to the bronchial submucosa: identification by chest CT. Chest 1998;114:1217-20. [Crossref] [PubMed]
- Fournel C, Bertoletti L, Nguyen B, et al. Endobronchial metastases from colorectal cancers: natural history and role of interventional bronchoscopy. Respiration 2009;77:63-9. [Crossref] [PubMed]
- Choi HS, Kim SY, Choi CW, et al. Use of bronchoscopic electrocautery in removing an endotracheal metastasis. Lung Cancer 2007;58:286-90. [Crossref] [PubMed]
- Lee M, Lee YK, Jeon TJ, et al. A case of tracheal metastasis in colon cancer: detection with 18F-FDG PET/CT. Clin Nucl Med 2015;40:91-2. [Crossref] [PubMed]
- Tsuboi R, Oki M, Saka H, et al. Rigid bronchoscopic intervention for endobronchial metastasis of renal cell carcinoma. Respir Investig 2016;54:250-4. [Crossref] [PubMed]


