Induction chemoradiotherapy using docetaxel and cisplatin with definitive-dose radiation followed by surgery for locally advanced non-small cell lung cancer
Introduction
Optimal management of locally advanced non-small cell lung cancer (LA-NSCLC) remains controversial and challenging. Based on the results of two large randomized phase III trials, which failed to demonstrate the benefit of addition of surgery, definitive chemoradiotherapy (CRT) is adopted as the standard treatment for patients with LA-NSCLC (1,2). On the other hand, pulmonary resection after induction CRT is one of the treatment options considered in selected patients (1), and we have previously reported the favorable outcome of induction CRT followed by surgery using docetaxel and cisplatin on the clinical outcome (3).
Although radiation doses of equal or more than 60 Gy are often used in patients receiving definitive CRT (4-6), lower doses (40 to 50 Gy) are mainly adopted in cases scheduled for induction CRT, considering the safety of the subsequent surgery (7-9). We usually use the radiation dose of 46 Gy in the induction setting. However, higher radiation doses of up to 60 Gy or higher, which were referred to as definitive-dose (DD) radiation, have occasionally been used in some cases where the treatment response is inadequate and cases where complete resection is expected to be difficult.
In general, the postoperative (PO) complication rate is reported to be higher in cases undergoing pulmonary resection after CRT using DD radiation (4-6) than after CRT with lower-dose radiation (10,11). By contrast, several studies in the 2000s have reported that the use of DD radiotherapy as induction CRT prior to surgery was not associated with an increased risk of the surgery (12-14). However, the safety and clinical outcomes of induction CRT using DD radiation has not been closely investigated. In addition, the chemotherapy regimens used in these reported studies were varied, and there are no reports of the studies of cases in which a unified chemotherapy regimen was used. To the best of our knowledge, this is the first report of investigation of the safety and clinical outcomes of induction CRT followed by surgery in LA-NSCLC patients receiving a unified chemotherapy regimen of third generation chemotherapy, docetaxel and cisplatin, which is one of the standard regimens used in patients receiving CRT, with DD radiation or non-DD radiation.
Methods
Patient selection and evaluation
A total of 125 patients with LA-NSCLC underwent induction CRT followed by radical pulmonary resection between January 1999 and December 2014 at Okayama University Hospital, Okayama, Japan. Among these, 110 patients who received induction CRT with docetaxel and cisplatin as the chemotherapy regimen were enrolled in this study. The medical records of the enrolled patients were retrospectively reviewed. The International Association of the Study of Lung Cancer tumor, node and metastasis staging system for NSCLC, 7th edition, was used for disease staging (15). The clinical stage of the disease was determined by chest radiography, bronchoscopy, computed tomography (CT) of the chest and abdomen, magnetic resonance imaging of the brain, and a radionuclide bone scan or 18F-fluorodeoxyglucose positron emission tomography-CT (16).
Informed consent was obtained from the patient along with the study protocol approved by the Institutional Review Board/Ethical Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama University Hospital (permission number: Eki1055).
Induction treatment, surgery and adjuvant treatment
The details of the patient inclusion criteria for this treatment setting are provided in our previous report (17). The induction CRT consisted of docetaxel and cisplatin chemotherapy with concurrent thoracic radiation, as described previously (18). In brief, docetaxel (40 mg/m2) was administered by intravenous injection followed by cisplatin (40 mg/m2) administered via the same route before radiotherapy on days 1 and 8. The chemotherapy was repeated at 3- or 4-week intervals. Radiotherapy was started on the first day of chemotherapy and a total radiation dose of 40 or 46 Gy (1) was planned, in principle, to be administered in conventional fractional doses of 2 Gy/day.
After receiving a radiation dose of around 40 Gy, the response in the patients was evaluated by image analysis including chest radiography and CT. The radiological response was classified into four categories, as described in previous studies (17,18). Patients without progressive disease were scheduled to undergo surgery about 6 to 8 weeks after completion of the induction CRT, based on a consensus among the oncologist, radiologist, and thoracic surgeon. On the other hand, in the occasional cases where incomplete resection was expected even after completion of 46 Gy irradiation because of severe tumor invasion to surrounding structures whose resection would be too invasive to preserve a sufficient surgical margin, additional radiotherapy up to a total radiation dose of 60 Gy or higher without interruption of irradiation due to estimation at the dose of 40 Gy was occasionally undertaken in an attempt to increase the efficacy of local control. The additional radiation was administered to the boost volume, including the sites of the primary tumor and the involved lymph nodes. The boost volume was treated with a pair of oblique fields to keep the cumulative radiation dose to the spinal cord at less than 46 Gy. After the additional radiation, patients who did not have unresectable lesions and who had a good general condition were scheduled to undergo surgery. Patients who were considered inoperable even after 60 Gy irradiation underwent follow-up without following surgery and were excluded from this study.
The surgical procedure employed was determined on the basis of the disease extent, and included lobectomy, which was the most preferred, bilobectomy, sleeve resection, or pneumonectomy with complete ipsilateral mediastinal and subcarinal nodal dissection as previously described (3). Resection with reconstruction of the chest wall and/or major vessels was performed where necessary. The bronchial stump or anastomosis was basically covered with pericardial fat tissue, greater omentum, or an intercostal muscle pedicle flap.
The pathological complete response (CR) to the induction CRT was defined as no viable cells seen on the tumor site or any of the resected lymph nodes (12). Any PO treatment was left to the physician’s discretion. If there was any apparent residual tumor, or viable cells were found in the surgically resected specimens, PO adjuvant chemotherapy was given as much as possible. After completion of the entire treatment course, the patients were followed up according to our follow-up protocol, which was described in a previous report (3).
Survival and statistical analysis
The baseline characteristics of the DD and non-DD groups were compared by the t-test for continuous variables and the Fisher’s exact test for categorical variables, as appropriate. The overall survival (OS) was calculated from the date of start of treatment until the date of death or the last follow-up, and the cancer-specific recurrence-free survival (RFS) was calculated from the date of start of treatment until the date of confirmation of cancer recurrence. Kaplan-Meier curves were drawn for evaluating the survival and compared among groups using the log-rank test. To control for variables potentially confounding the association between the therapeutic modality and survival, a propensity score (PS)-matched analysis was performed. We calculated the PS by logistic regression based on available factors that were considered to be potentially associated with the patient selection. Fisher’s exact test was performed using R v3.2.5 software (http://www.r-project.org). Other tests were performed using the JMP v11 software (SAS Institute Inc., Cary, NC). In each of the analyses, a probability value of less than 0.05 was considered as denoting statistical significance.
Results
Patient characteristics and induction CRT
Among the 110 patients enrolled, 99 received non-DD radiotherapy (less than 60 Gy; non-DD group) and 11 patients received DD radiotherapy (60 Gy or higher; DD group). The patient characteristics are shown in Table 1. Cases with an advanced clinical stage of the disease were more frequent in the DD group than in the non-DD group (0% vs. 10% for IIB, 36% vs. 66% for IIIA, 64% vs. 24% for IIIB, respectively) (P=0.033). No significant differences were found in any of the other patient characteristics between the two groups.
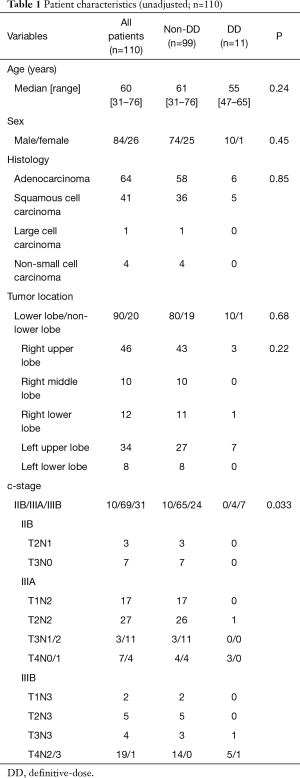
Full table
The details of induction CRT are shown in Table 2. The median doses of radiation were 46 and 60 Gy in the non-DD and DD groups, respectively. No significant difference was found in the rate of the dose reduction or interruption of chemotherapy, or the radiological response between the two groups.
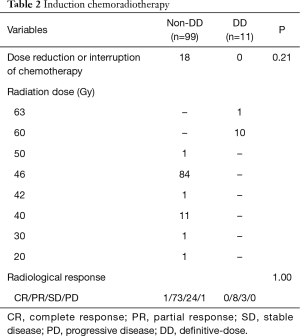
Full table
Surgery and pathological response
There was no significant difference in the operation types or the extent of lymph node dissection between the two groups (Table 3). On the other hand, the rate of combined resection and the intra-operative blood loss tended to be higher in the DD group than they were in the non-DD group (P=0.051 for combined resection and P=0.054 for blood loss). There was no significant difference in the operation time between the two groups (P=0.15). Other than one patient of the non-DD group who exhibited pathologic incomplete tumor resection with pleural dissemination that was diagnosed by histopathology after the surgery, the remaining 109 patients showed pathologic complete tumor resection, and no significant difference in the complete resection rate was observed between the two groups (P=1.00).
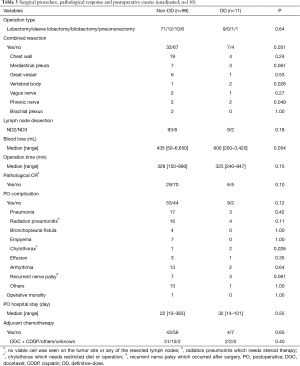
Full table
The pathological CR rate tended to be higher in the DD group (55%) than in the non-DD group (29%, P=0.10).
PO complications and adjuvant therapy
There was no significant difference in the PO complication rate between the non-DD and DD groups (56% vs. 82%, P=0.12) (Table 3). Among the PO complications, chylothorax occurred significantly more frequently in the DD group than in the non-DD group (P=0.026). There was no statistical difference in the rate of radiation pneumonitis between the two groups. One patient of the non-DD group died because of empyema complicating bronchopleural fistula (mortality rate: 1.0% in the non-DD group vs. 0% in the DD groups, P=1.00).
As for adjuvant therapy, 43% and 36% of the non-DD and DD groups, respectively, received PO adjuvant chemotherapy.
Survival and pattern of relapse
At a median follow-up duration of 45 months, 20 and 3 patients of the non-DD and DD groups, respectively, died of the primary disease, and 7 and 1 patients in the two groups, respectively, died of other causes.
Thirty-five patients (35%) of the non-DD group and two patients (18%) of the DD group developed disease relapse. We classified the pattern of disease recurrence as local recurrence (surgical margin, intrapulmonary, regional lymph node, and/or pleural cavity) or distant metastasis. In the non-DD group, the initial recurrence was classified as local recurrence alone in 7 patients, as distant metastasis alone in 22 patients, and as including both recurrence patterns in 6 patients. On the other hand, in the DD group, the initial recurrence was classified as a local recurrence in 1 patient and as including both patterns of recurrence in the other. No significant differences in the recurrence patterns were observed between the two groups (P=0.16).
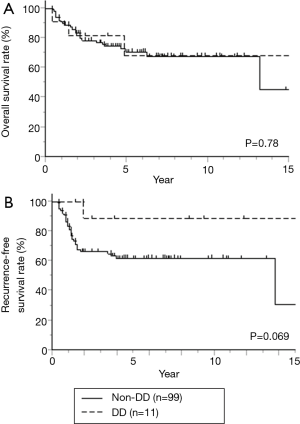
The 3- and 5-year OS rates were 78% and 71% in the non-DD group, vs. 82% and 68% in the DD group, respectively (P=0.78) (Figure 1A). The 3- and 5-year RFS rates were 66% and 62% in the non-DD group, vs. 89% and 89% in the DD group, respectively (Figure 1B), and RFS tend to be higher in the DD group than in the non-DD group (P=0.069).
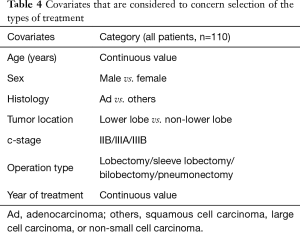
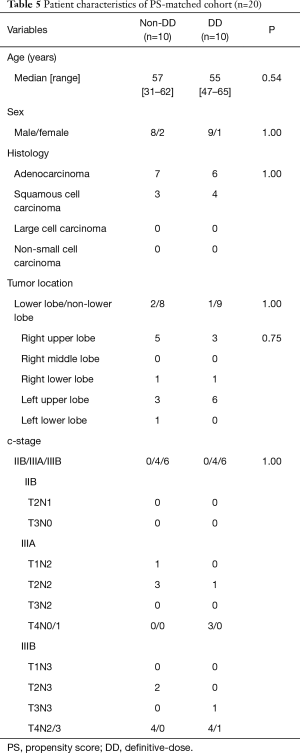
PS-matched analysis
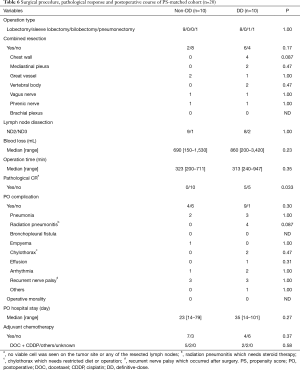
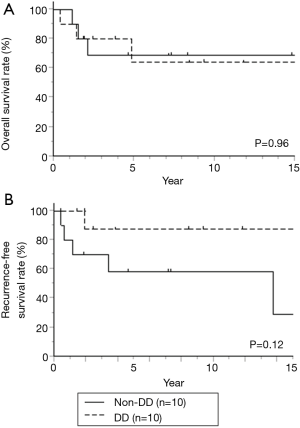
Seven factors that were included in the PS calculation were age, sex, histology, tumor location, clinical stage, operation type, and year of treatment (Table 4). Among the 110 patients, 10 patients each were extracted from each group by PS-matching. The patient characteristics were matched as shown in Table 5. After adjustments, the pathological CR rate was found to be significantly higher in the DD group (50%) than in the non-DD group (0%, P=0.033) (Table 6). There was no significant difference in the mortality rate (0% in the both groups) or the PO complication rate between the two groups. Among PO complications, radiation pneumonitis tended to be more frequent in the DD group than in the non-DD group (P=0.087). No significant difference in OS or RFS was observed between the two groups (Figure 2).
Full table
Full table
Full table
Discussion
Our study revealed that the pathologic CR rate was significantly higher in the DD group than in the non-DD group (P=0.033) after adjustment based on the PS. This finding was consistent with a previous small study that Cerfolio et al. investigated the clinical impact of induction CRT followed by surgery comparing high and low dose radiation (12). However, the pathologic CR rate in the DD group in our study was very high (55%), even compared with that in their study (28%). The high pathological CR rate in our study may be dependent on the fact the patients who were considered inoperable after 60 Gy irradiation were excluded from our study, and we used cisplatin based regimen which was reported to be superior in the local control than carboplatin based regimen (19). Regarding the prognosis, no significant impact was observed in the DD group as compared to the non-DD group. However, as for the prognostic impact of pathologic CR, previous studies, larger in scale than our present study, have shown the existence of a correlation between a higher pathological CR rate and favorable prognosis in patients who underwent induction CRT followed by surgery (20,21). In fact, only one patient relapsed within 2 years in the DD group. This may be caused by effectiveness of local control in the DD group.
In this study, the PO complication rates were higher in the DD group (82%) than in the non-DD group (56%, P=0.12). However, severe PO complications such as bronchopleural fistula or empyema were not observed in the DD group. Regarding the individual PO complication, the number of patients developing radiation pneumonitis tended to be higher in the DD group than in the non-DD group, after adjustment based on the PS. According to some previous studies, the radiation dose escalation in patients receiving induction CRT with DD radiation prior to surgery was not associated with an increased risk of radiation pneumonitis, although various chemotherapy regimens had been used in the enrolled patients in these studies (12,13). On the other hand, some studies have reported radiation dose escalation in CRT as a risk factor for the development of radiation pneumonitis (22-24). Furthermore, use of taxane-based regimens, as in this study, has also been reported as a risk factor for the development of radiation pneumonitis in CRT (22,25). Fortunately, none of our cases developed severe radiation pneumonitis, and the condition was managed appropriately, even though steroid treatment was needed in some cases. However, “destroyed lung”, which occasionally developed after radiation therapy decreases the pulmonary function and can be responsible for chronic secondary complications, such as pulmonary aspergillosis (26). Therefore, careful long-term follow-up for radiation therapy-related complications is required in patients receiving radiation therapy.
This study was a non-randomized study, and contains considerable biases in respect of the patient selection: for example, (I) clinical stage: DD radiotherapy was applied significantly more frequently for patients with advanced stage of the disease; (II) operation type: aggressive operations (combined resection of other organs) were borderline more frequent in the DD group; and (III) tumor location: the number of tumors of lower lobe origin was less than half in the DD group as compared to that in the non-DD group. As for tumor location, the V20 value, a predictor of the risk of radiation pneumonitis (27), is higher in tumors of lower lobe origin as compared to tumors of non-lower lobe origin, suggesting the less chance of DD radiotherapy for tumors located in the lower lobe from the view point of radiation field. Furthermore, it has been reported that LA-NSCLC patients treated by induction CRT prior to surgery with tumors of lower lobe origin showed a significantly worse prognosis than those with tumors of non-lower lobe origin (28). All of these biases might influence on the results of our current study. In order to reduce these biases inherent in this series, we performed PS-matched analysis including all three above-mentioned factors, and the results were almost similar to those obtained before PS-matching. Nevertheless, a large-scale retrospective study or a well-designed prospective study is necessary to endorse our conclusions.
In conclusion, our study showed that induction CRT using docetaxel and cisplatin with radiation doses of 60 Gy or higher is safe, although appropriate caution is required in view of the possibly higher risk related to radiation as a PO complication in patients receiving DD radiation. DD radiation was associated with an increased pathologic CR rate as compared with non-DD radiation, suggesting the possibility that surgery after CRT with DD dose may be one of treatment option for LA-NSCLC.
Acknowledgements
We thank Drs. Yusuke Ogoshi, Eisuke Kurihara, and Yuta Takahashi of the Department of Thoracic Surgery, Okayama University Hospital, Okayama, Japan, for collecting the patient data.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board/Ethical Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama University Hospital (Eki1055).
References
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Toyooka S, Kiura K, Takemoto M, et al. Long-term outcome of induction chemoradiotherapy with docetaxel and cisplatin followed by surgery for non-small-cell lung cancer with mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 2012;14:565-9. [Crossref] [PubMed]
- Segawa Y, Kiura K, Takigawa N, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol 2010;28:3299-306. [Crossref] [PubMed]
- Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol 2010;28:3739-45. [Crossref] [PubMed]
- Sekine I, Nokihara H, Sumi M, et al. Docetaxel consolidation therapy following cisplatin, vinorelbine, and concurrent thoracic radiotherapy in patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol 2006;1:810-5. [Crossref] [PubMed]
- Rusch VW, Benfield JR. Neoadjuvant therapy for lung cancer: a note of caution. Ann Thorac Surg 1993;55:820-1. [Crossref] [PubMed]
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [Crossref] [PubMed]
- Kraut MJ, Rusch VW, Crowley JJ, et al. Induction chemoradiotherapy plus surgical resection is a feasible and highly effective treatment for Pancoast tumors: initial results of SWOG 9416 (Intergroup 0160) Trial. Proc Am Soc Clin Oncol 2000;19:487a.
- Fowler WC, Langer CJ, Curran WJ Jr, et al. Postoperative complications after combined neoadjuvant treatment of lung cancer. Ann Thorac Surg 1993;55:986-9. [Crossref] [PubMed]
- Deutsch M, Crawford J, Leopold K, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy with thoracotomy in the treatment of clinically staged IIIA non-small cell lung cancer. Cancer 1994;74:1243-52. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Spencer SA, et al. Pulmonary resection after high-dose and low-dose chest irradiation. Ann Thorac Surg 2005;80:1224-30; discussion 1230. [Crossref] [PubMed]
- Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann Thorac Surg 2004;78:1200-5; discussion 1206. [Crossref] [PubMed]
- Krasna MJ, Gamliel Z, Burrows WM, et al. Pneumonectomy for lung cancer after preoperative concurrent chemotherapy and high-dose radiation. Ann Thorac Surg 2010;89:200-6; discussion 206. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 52-3. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Katayama H, Ueoka H, Kiura K, et al. Preoperative concurrent chemoradiotherapy with cisplatin and docetaxel in patients with locally advanced non-small-cell lung cancer. Br J Cancer 2004;90:979-84. [Crossref] [PubMed]
- Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 2007;99:847-57. [Crossref] [PubMed]
- Kim AW, Liptay MJ, Bonomi P, et al. Neoadjuvant chemoradiation for clinically advanced non-small cell lung cancer: an analysis of 233 patients. Ann Thorac Surg 2011;92:233-41; discussion 241-3. [Crossref] [PubMed]
- Lococo F, Cesario A, Margaritora S, et al. Long-term results in patients with pathological complete response after induction radiochemotherapy followed by surgery for locally advanced non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;43:e71-81. [Crossref] [PubMed]
- Onishi H, Kuriyama K, Yamaguchi M, et al. Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no good survival due to radiation pneumonitis. Lung Cancer 2003;40:79-84. [Crossref] [PubMed]
- Rodrigues G, Lock M, D'Souza D, et al. Prediction of radiation pneumonitis by dose - volume histogram parameters in lung cancer--a systematic review. Radiother Oncol 2004;71:127-38. [Crossref] [PubMed]
- Bradley JD, Moughan J, Graham MV, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 2010;77:367-72. [Crossref] [PubMed]
- Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444-50. [Crossref] [PubMed]
- Citak N, Sayar A, Metin M, et al. Results of surgical treatment for pulmonary aspergilloma with 26 cases in six years: a single center experience. Tuberk Toraks 2011;59:62-9. [Crossref] [PubMed]
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2005;61:318-28. [Crossref] [PubMed]
- Shien K, Toyooka S, Soh J, et al. Lower lobe origin is a poor prognostic factor in locally advanced non-small-cell lung cancer patients treated with induction chemoradiotherapy. Mol Clin Oncol 2015;3:706-12. [Crossref] [PubMed]


