Fluid resuscitation targeting sepsis-induced cardiovascular dysfunction: severity of disease as effect modifier
Fluid resuscitation is the cornerstone of resuscitation in patients with sepsis and septic shock. According to a recent retrospective cohort study of patients with a diagnosis of sepsis, respiratory events, among others, were the strongest predictors of mortality in hospitalized patients (1). Preventing development of nosocomial adult respiratory distress syndrome (ARDS) can contribute to improving survival in this cohort of patients (2). Strategies hereto comprise, apart from hemodynamic resuscitation for sepsis, prophylactic treatment of ARDS with appropriate medications and timely antimicrobials. Furthermore, restrictive transfusion practices help avoid transfusion-related adverse events, and precautionary measures must be in place to prevent aspiration. Adhering to sepsis guidelines has resulted in lowering morbidity and mortality rates, but they continue to be high (3). To reduce shock duration and associated poor survival in sepsis, prompt and adequate fluid resuscitation is recommended (4).
Whereas fluid resuscitation is of paramount importance in treating sepsis, inappropriate administration in terms of type, dose, and duration can result in damage to organs leading to death so that these aspects must be carefully kept in mind during therapy (4). Randomized controlled trials (RTCs), however, have shown that despite adherence to recommended guidelines and achieving hemodynamic stability in early goal-directed therapy (EGDT), there were varying survival outcomes (5). In a total of 3,723 sepsis patients analyzed by three largest RCTs (6), survival was not better in patients receiving EGDT as compared to those receiving standard care. A subgroup analysis revealed that EGDT conferred survival advantage in 370 patients with chronic obstructive pulmonary disease, but in 117 patients with severe chronic liver disease, EGDT was associated with increased mortality (6). These results suggest that in dealing with heterogeneous septic patient populations, one size fits all approach to EGDT is unlikely to work. Very little, however, is known on whether and how the presence of different comorbidities in sepsis patients affects achievement of hemodynamic stability via fluid administration and thus survival outcomes. Further studies are needed in selected patient populations including those with thoracic diseases.
In a study aimed at finding an explanation for varying outcomes of EGDT in sepsis patients, 19,998 patients from 31 observational and 6 randomized trials of EGDT in sepsis were analyzed (5). Lower risk of death after EGDT was revealed in observational studies but not in RCTs, although the patients were comparable in all relevant parameters including hemodynamic goal achievement. The only factor that could explain the difference in divergent mortality outcomes between observational studies and RCTs was co-administration of antibiotics with EGDT. This was a finding in adult as well as pediatric patient populations in the observational studies but not in the RCTs. Interestingly, the more severe the disease, the higher was the rate of mortality in patients receiving EGDT.
Recently, in Intensive Care Medicine, Marik et al. (7) retrospectively analyzed the 2013 U.S. Premier Hospital Discharge database, a large national database of 23,513 ICU patients with severe sepsis and septic shock and reported on the relationship between the volume of fluid administration and in-hospital mortality. The Premier Hospital Discharge database is an automated administrative database containing all billed items, date-stamped medications and laboratory, diagnostic and therapeutic services for each patient. The difference between actual and expected mortality was assessed in relation to the amount of fluid administered on day 1. Not surprisingly, fluid input was higher in patients who were ventilated and in shock than in patients without these diagnoses (7). Previous cohort studies have reported similar findings of higher amounts of early fluid input in patients with greater disease severity (8-10). Importantly, in the study by Marik et al. (7), actual mortality exceeded the expected mortality for patients who received greater amounts of fluids on day 1, particularly in those receiving highest fluid volumes when patients were ventilated, in shock, or both. All these findings confirm the hypothesis that liberal EGDT may be particularly harmful in some patients, especially those in shock and/or on ventilation who are sicker, need more fluids, and have higher mortality (11).
There are several limitations inherent in the study of Marik and co-workers (7) including its retrospective observational design that does not permit any conclusion on causality. Another limitation is the fact that day 1 was defined and calculated as a calendar day, not 24 h, which is of particular relevance since the amount of resuscitation fluid administered on day 1 was the parameter of interest for the proposed fluid volume cut-off on day 1 of 5 L. Assuming that statistics have successfully adjusted for this important bias (leading to the exclusion of centers and patients), whether fluid administration of more than 5 liters on day 1 is associated with increased mortality in patients in shock and/or on ventilation depends on the adequacy of the actual versus predicted mortality comparisons. Thus, it is difficult to inform clinical practice and define or confirm a proposed (12) “safe” day 1 volume for EGDT in sepsis. In addition, unmeasured variables or interventions are always a possible source of bias in observational studies; for example, it is known that hypoproteinemia is significantly correlated with fluid retention and weight gain, development of ARDS and poor respiratory outcome, and mortality in patients with sepsis (13). Yet in the study by Marik et al. (7), no information is given on hypoproteinemia as a particular risk factor. It is also not clear which types of fluids had been administered, whether they were 0.9% saline, balanced crystalloids, semisynthetic colloids or albumin; their use has seen dramatic changes in recent years due to new evidence on their efficacy and safety (14). Both hypoproteinemia and administration of colloids for volume resuscitation may be of particular relevance in septic shock patients complicated by acute respiratory failure (15,16).
Achieving positive fluid balance on the first day in the ICU by means of fluid resuscitation was negatively associated with hospital mortality. Fluid gain on the second and third days of ICU stay was associated with disease severity, and this was positively correlated with hospital mortality. Thus, disease severity might affect the benefits of fluid resuscitation in terms of survival in severe sepsis and septic shock (17,18).
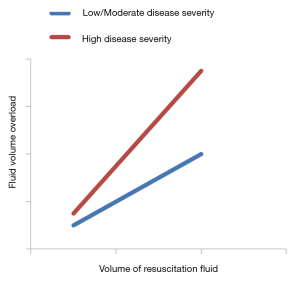
It is not known if EGDT further increases mortality compared with sick controls without EGDT due to effect heterogeneity that is determined by disease severity (Figure 1). In order to test EGDT for effect modification and treatment interactions, survival outcomes in the Protocolized Care for Early Septic Shock (ProCESS) trial were analyzed in relation to inflammatory, coagulation, oxidative stress, and tissue hypoxia biomarkers measured in patients enrolled (19). Whereas protocol-based resuscitation had no effects on baseline coagulation, oxidative stress, and tissue hypoxia biomarkers, patients with lower baseline concentrations of inflammatory biomarkers seemed to benefit from it. This raises the possibility that EGDT may prove to be harmful to patients with high disease severity and elevated biomarkers of inflammation (19).
Other biomarkers not yet sufficiently investigated in association with effect heterogeneity of EGDT may be important as well. Thus, it is known from acute respiratory failure of ARDS patients with or at risk for the syndrome after new onset of fever that hypoalbuminemia may be of greater value than C-reactive protein in predicting and monitoring the severity and course of disease (20). In patients with ARDS, low levels of aldosterone, a marker of effective circulating volume, may identify those in whom restrictive fluid management can contribute to survival benefit (21).
Sepsis, severe sepsis, and septic shock represent increasingly severe systemic inflammatory responses to infection and may be complicated by acute respiratory failure. EGDT of sepsis and septic shock could benefit patients with low disease severity; the fixed bundle protocol approach to treating patients with severe disease might well turn out to be harmful (22). Patients with high disease severity may, therefore, require a more individualized and flexible resuscitation approach.
Effect modification by disease severity of therapies targeting sepsis-induced organ and immune dysfunction has long been investigated (23). It has been reported, for instance, that sepsis patients with high disease severity and high risk of death, with or without bleeding complications, may benefit from treatment with high-dose antithrombin III, with an even higher survival advantage if no heparin is administered concomitantly (24). EGDT may be another therapeutic intervention whose efficacy and safety will depend on particular pathophysiological and pharmacological mechanisms related to disease severity (22). In patients with acute lung injury with a more permeable endothelium, for instance, liberal fluid administration can exacerbate organ dysfunction, and lead to the development of acute respiratory failure from ARDS, whereas a conservative approach to fluid management can improve lung function without jeopardizing the function of non-pulmonary organs (25). The work by Marik et al. (7) confirms uncertainties in hemodynamic management of sepsis and high disease severity, when the self-reinforcing pathophysiologic processes involved in sepsis may adversely interact with pharmacodynamic properties of fluid therapies. Such undesired interaction may cause, among others, endothelial injury resulting in activation of monocytes and granulocytes, endothelial barrier breakdown, immunothrombosis, and disseminated intravascular coagulation (26,27). In order to enhance the prospects of therapeutic efficacy for new treatment strategies, the use of clinical and biological criteria to select and phenotype patients with sepsis for clinical trials thus reducing patient heterogeneity will need to be improved.
Acknowledgements
The authors wish to thank Rajam Csordas for critical reading.
Footnote
Conflicts of Interest: CJ Wiedermann has received lecture fees and/or travel cost reimbursements from the Plasma Protein Therapeutics Association (PPTA), Kedrion, CSL Behring, Grifols and Baxter, and remuneration for consulting from CSL Behring, Grifols, and Daiichi Sankyo. S Dunzendorfer has no conflicts of interest to declare.
References
- Elfeky S, Golabi P, Otgonsuren M, et al. The epidemiologic characteristics, temporal trends, predictors of death, and discharge disposition in patients with a diagnosis of sepsis: a cross-sectional retrospective cohort study. J Crit Care 2017;39:48-55. [Crossref] [PubMed]
- Beitler JR, Goligher EC, Schmidt M, et al. Personalized medicine for ARDS: the 2035 research agenda. Intensive Care Med 2016;42:756-67. [Crossref] [PubMed]
- Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3-12. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Kalil AC, Johnson DW, Lisco SJ, et al. Early goal-directed therapy for sepsis: A novel solution for discordant survival outcomes in clinical trials. Crit Care Med 2017;45:607-14. [Crossref] [PubMed]
- PRISM Investigators, Rowan KM, Angus DC, et al. Early, goal-directed therapy for septic shock – a patient-level meta-analysis. N Engl J Med 2017;376:2223-34. [Crossref] [PubMed]
- Marik PE, Linde-Zwirble WT, Bittner EA, et al. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017;43:625-32. [Crossref] [PubMed]
- Smith SH, Perner A. Higher vs. lower fluid volume for septic shock: clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care 2012;16:R76. [Crossref] [PubMed]
- Carlsen S, Perner A. Initial fluid resuscitation of patients with septic shock in the intensive care unit. Acta Anaesthesiol Scand 2011;55:394-400. [Crossref] [PubMed]
- McIntyre LA, Fergusson D, Cook DJ, et al. Resuscitating patients with early severe sepsis: a Canadian multicentre observational study. Can J Anaesth 2007;54:790-8. [Crossref] [PubMed]
- Genga K, Russell JA. Early liberal fluids for sepsis patients are harmful. Crit Care Med 2016;44:2258-62. [Crossref] [PubMed]
- Levy MM. Early goal-directed therapy: Sorting through confusion. Lung India 2015;32:435-6. [Crossref] [PubMed]
- Mangialardi RJ, Martin GS, Bernard GR, et al. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med 2000;28:3137-45. [Crossref] [PubMed]
- Hammond NE, Taylor C, Finfer S, et al. Patterns of intravenous fluid resuscitation use in adult intensive care patients between 2007 and 2014: an international cross-sectional study. PLoS One 2017;12:e0176292. [Crossref] [PubMed]
- Chang DW, Huynh R, Sandoval E, et al. Volume of fluids administered during resuscitation for severe sepsis and septic shock and the development of the acute respiratory distress syndrome. J Crit Care 2014;29:1011-5. [Crossref] [PubMed]
- Uhlig C, Silva PL, Deckert S, et al. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care 2014;18:R10. [Crossref] [PubMed]
- Shum HP, Lee FM, Chan KC, et al. Interaction between fluid balance and disease severity on patient outcome in the critically ill. J Crit Care 2011;26:613-9. [Crossref] [PubMed]
- Genga KR, Russell JA. How much excess fluid impairs outcome of sepsis? Intensive Care Med 2017;43:680-2. [Crossref] [PubMed]
- Kellum JA, Pike F, Yealy DM, et al. Relationship between alternative resuscitation strategies, host response and injury biomarkers, and outcome in septic shock: analysis of the Protocol-Based Care for Early Septic Shock Study. Crit Care Med 2017;45:438-45. [Crossref] [PubMed]
- Hoeboer SH, Oudemans-van Straaten HM, Groeneveld AB. Albumin rather than C-reactive protein may be valuable in predicting and monitoring the severity and course of acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new onset fever. BMC Pulm Med 2015;15:22. [Crossref] [PubMed]
- Semler MW, Marney AM, Rice TW, et al. B-type natriuretic peptide, aldosterone, and fluid management in ARDS. Chest 2016;150:102-11. [Crossref] [PubMed]
- Kalil AC, Kellum JA. Is early goal-directed therapy harmful to patients with sepsis and high disease severity? Crit Care Med 2017;45:1265-7. [Crossref] [PubMed]
- Minneci PC, Deans KJ, Cui X, et al. Antithrombotic therapies for sepsis: a need for more studies. Crit Care Med 2006;34:538-41. [Crossref] [PubMed]
- Wiedermann CJ, Hoffmann JN, Juers M, et al. High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: efficacy and safety. Crit Care Med 2006;34:285-92. [Crossref] [PubMed]
- Wiedemann HP, Wheeler AP, Bernard GR, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75. [PubMed]
- Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016;353:i1585. [Crossref] [PubMed]
- Feistritzer C, Wiedermann CJ. Effects of anticoagulant strategies on activation of inflammation and coagulation. Expert Opin Biol Ther 2007;7:855-70. [Crossref] [PubMed]


