Robotic portal lobectomy, surgery through a virtual thoracotomy
Resection for lung cancer remains the most effective mechanism to offer a chance at cure from a potentially devastating disease. We have known this fact for almost an entire century ever since the first resection by pneumonectomy for lung cancer was performed by Dr. Evarts Graham (1). Despite recent advances in nonsurgical technologies such as stereotactic radiation and percutaneous ablation, resection of the cancer with an adequate margin along with the anatomical unit of lung in which it resides including lymphatics, vessels and parenchyma remains the gold standard of care, against which competing modalities must be judged. Whether this “anatomical unit” is 1 lobe, 2 lobes, one or more segments, or even the whole lung depends on many factors including pulmonary functional status, comorbidities, location of tumor, size and histology. Increasing evidence of possible equivalence of sublobar resection to lobectomy is emerging especially for small or subsolid tumors associated with less aggressive lepidic adenocarcinomas (2,3).
Regardless of the extent of lung resection, the surgical approach to the lung can be classified as either by thoracotomy (with rib spreading) or by endoscopy (without). Rib-spreading thoracotomy has been the standard procedure and provides excellent exposure of the hilum in addition to allowing natural two-handed surgical techniques in dissection. However, in many studies it has also been associated with higher incidence of morbidity and even less favorable outcomes than minimally invasive approaches. Postoperative morbidities often occur when patients have intercostal neuralgic pain causing poor respiratory effort leading to atelectasis and pneumonia. In addition, up to 20% of patients will have chronic post-thoracotomy pain that is resistant to most forms of treatment. Here lies the crux of the matter and why there is an enormous interest in developing a better way of doing this operation.
Non-rib spreading video-assisted thoracoscopic surgery (VATS) has been used to describe minimally invasive thoracic procedures. Such procedures were found not only to be feasible but also associated with better outcomes (4-8). However, as any surgeon who has done VATS lobectomy knows, it is a fundamentally different operation than that done through thoracotomy. This is primarily due to the different viewing angle which is necessarily anterior to the hilum. The approach is therefore, usually anterior to posterior, with division of the fissure last. As surgeons have gained more experience with this approach some find that they are able to adapt it to increasingly more difficult situations. However, it is ideally indicated for peripheral small tumors not associated with significant hilar adenopathy (8). Otherwise, the straight instruments do not allow easy manipulation of the lung and the 2-dimensional camera prevents good depth perception, preventing the surgeon from instinctively judging the necessary maneuvers needed for difficult dissection.
More recently, robotic technology has entered the arena of minimally invasive surgery. The benefits of dexterous dissection and manipulation in a confined space make it ideal for dissection in the chest. In the thoracic cavity, the ability of the surgeon to handle and manipulate the pulmonary hilar vessels and structures with excellent 3-dimensional visualization allows the safe conduct of the operation. Robotic surgery has allowed fine dissection of lymph nodes with better precision than traditional endoscopic techniques. However, in this author’s opinion one of the main advantages of robotic thoracoscopy is that it almost perfectly replicates the open approach. The viewing angle is top down, not from the side. The surgeon has an instrument on each side of the camera, i.e., it is a natural surrogate for the natural human anatomy with a hand on each side of the eyes. The wristed nature of the instruments perfectly allows unlimited manipulation of the tissues and the ability to perform fine sharp dissection as we were taught to do so with open lobectomy. This is perhaps nowhere more important than when operating on the pulmonary vessels or when attempting to extirpate mediastinal lymph nodes. In fact, robotic portal lobectomy is simply an open procedure done through small holes, i.e., it is a “virtual thoracotomy”. As such, it gives the surgeon the visual illusion of looking at the lung through an open chest and of being able to address the anatomy in a natural bimanual wristed technique. This has resulted in surgeons who were critical of VATS for various reasons finding they are able to easily adopt this new technology even for advanced cases. Unpublished reports suggest that up to 20% of lobectomies in the US are currently done robotically. An interesting fact, since the first published reports were only about 10 years ago.
As robotic-assisted thoracic surgery (RATS) appeared on the scene, so too has the name been used to describe any operation where the robot was used. Some surgeons perform the operation completely through ports. Others make a utility incision through which the assistant has both visual and manual access to the field. Yet others may use the robot for some of the procedure and VATS or thoracotomy for the rest, the so called “hybrid” procedures. For this reason, a new nomenclature recently proposed classifying robotic thoracic procedures as either “robotic assisted” where the assistant uses a utility incision at the bedside or “robotic portal” where the only assistance is through a surgical port. The name RATS may therefore disappear giving way to terms such as RPL4 or RAL3 when describing a robotic lobectomy (9,10).
Dr. Chen and colleagues from Department of Thoracic Surgery, Ruijin Hospital in Shanghai provide a clear elegant example of a robotic portal 4 arm (RPL4) right middle lobectomy for a suspicious ground glass nodule that turned out to be a minimally invasive adenocarcinoma (11). Because of the tumor’s central location, it was not amenable to a preoperative biopsy or to sublobar resection. The patient had an uneventful postoperative course and was discharge on the 4th postoperative day. Although this is not a newly described procedure, the paper is beautifully illustrated and provides a comprehensive overview of their surgical program. Their patient care starts long before the day of surgery. The patient is instructed on respiratory training including breathing, coughing and expectoration after surgery, even on using a bedside commode. In the operating room, they have established a system that works for them including bed location, patient position, port placement, instruments used and team members available. Postoperatively, they have an ambulation and physical therapy protocol with early discharge when possible. These are the hallmarks of how to have a successful surgical program with excellent outcomes. Each of these items may often be taken for granted and are seldom reproduced as well as this paper illustrates.
The operative details are clearly outlined with superb illustrations and photos. Although, their port placement is not the only approach to this operation it is certainly conducive to excellent visualization and handling. They prefer to place the ports in multiple intercostal spaces, whereas the author of this article prefers to place all of the robotic ports in the 8th intercostal space whenever possible. This minimizes the possibility of causing more than one intercostal neuralgia. In addition, we prefer to place the assistant port subcostally, through the insertion of the diaphragm on the costal margin. This avoids having to remove a large specimen through an intercostal space causing intercostal nerve compression. Even pneumonectomies and large tumors can be removed in this fashion without the need for any rib spreading. Of course, the diaphragm must be reinserted when closing this incision using permanent suture attaching it back to the costal margin.
An important aspect when trying to understand a robotic procedure is to know which robot model was actually used for the procedure. Advances in the robotic system continue to develop and not all models are universally available. The newer Xi robot (currently only available in the US) provides certain advantages including 360-degree rotation of the arms, robotic vascular stapling and higher definition. This has allowed two major changes in how the robotic thoracic procedures can be performed. The first is the ability to dock the robot from the side of the patient instead of the head. Head docking makes it difficult for the anesthesiologist to access the patient’s head, e.g., to manipulate the endotracheal tube if necessary. With side docking, the patient’s head is clear and there is no need to rotate the bed away from the anesthesia cart. The second major benefit is robotic stapling where the surgeon is able to truly control what may be the most critical part of the procedure, dividing the hilar structures. This takes away one of the criticisms of robotic surgery. However, as anyone who has observed a robotic lobectomy with bedside stapling knows, the console surgeon is indeed in control, as much as in any open procedure, the surgeon performs the exposure of the vessel and helps guide the stapler around it. The assistant must carefully communicate with the surgeon, not make sudden moves and finally fire the stapler, much as they would do when assisting in a thoracotomy or VATS procedure. I believe that the described approach by the authors and their port placement works well for bed-side stapling whereas if the robotic stapler was used, the ports would need to be lower, i.e., in the 8th or 9th intercostal space.
The authors make two important comments in this article which are truly take-home messages. The first is that appropriate body position and incisions are key elements of this procedure. This is perhaps more important in robotic surgery than in any other. Once the robot is docked and the surgeon is at the console it is very difficult to change port placement or patient position. It is essential therefore, as these surgeons have done, to develop a clear understanding of the angles required by the robotic arms and the clearance provided by the spatial relationship of the ports to the anatomy of the patient and to one another. This comes with experience but once a system has been developed surgeons find that it is remarkable consistent and can be standardized to most patients.
The second important point they make is that the assistant should cooperate well with the surgeon and be experienced in both thoracoscopic and open surgery. Again, this is especially true for robotic surgery since the primary surgeon is actually not at the patient’s side. Perfect communication between the console surgeon and the bedside assistant is essential. Indeed the “assistant” is really the bedside surgeon. She or he must lead the surgical team and let the console surgeon know if any potential problems such as arm collision or difficulty with any aspect of their end of the procedure. Occasionally the angle between one of the arms and the assistant port is insufficient for safe stapling and this must be conveyed to the console surgeon. In an emergency, this same assistant must be able to handle the situation almost independently and this requires knowledge of thoracic surgery not just robotic techniques. It is important therefore, to constantly review different emergency scenarios with the entire team so that when it is necessary each individual in the room knows exactly what his or her role is. This author routinely announces a preprocedural “timeout” reviewing the role of the anesthesiologist to call for assistance, the circulating nurse to call for blood, the scrub nurse to start the undocking procedure and the assistant to perform whatever is necessary at the time e.g. holding pressure on a bleeder or making a thoracotomy (Table 1). Thoracoscopic and open instruments should be immediately available either open or in the room. Although the need for conversion will diminish with experience, it should remain as an expectation not a surprise for any busy thoracic program.

Full table
As robotic technology continues to evolve and as more medical device companies enter this arena, we are bound to see rapid advances in the not too distant future. The field of ideas is vast but certain needs come to mind. The ability to provide better haptic feedback for the console surgeon would eliminate one of the most often voiced concerns of non-robotic surgeons. Another example of possible upcoming advances is image overlay, essentially being able to overlay a reconstructed 3-dimensional study (e.g., CT scan or MRI) over the real-time video image and use this to identify important anatomical structures below the pleural surface such as the pulmonary arterial branches or a deep small nodule.
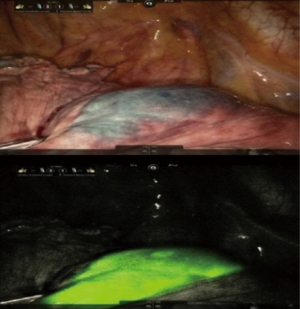
Another useful addition would be to add navigational technology to the robotic platforms. One recent publication by this author’s group describes incorporating electromagnetic navigational bronchoscopic localization of nodules by injecting them with indocyanine green (ICG) (12). This method benefits from the ability of the robot to use near infrared laser emission by specially equipped robotic cameras to identify the autofluorescent ICG-injected nodules. This allows the detection of small, deep or subsolid nodules that may be difficult to find otherwise (12) (Figure 1). Perhaps in the future we can incorporate navigational technology into the robotic platform and allow the robot itself to be directed to the target nodule.
Perhaps the most revolutionary change we can have in robotic surgical technology would be to make it more accessible. It continues to carry a hefty price tag and is not available to the vast majority of surgeons and their patients in the world. Making the robot more affordable will need more competition by manufacturers and academic institutions. As we have shown, robotic surgery may actually be profitable when it leads to better outcomes, shorter hospital stays and faster returns to work. It should therefore be preferentially selected for cases with high acuity such as thoracic procedures where these benefits will make a real difference. Using it for simple outpatient procedures such as sympathectomy or cholecystectomy may not be cost-effective at this time (13).
Robotic technology may allow more lung cancer patients to have minimally invasive thoracoscopic surgery instead of rib-spreading thoracotomy. With experience, it can be consistently used for more advanced cases such as bilobectomy, pneumonectomy, sleeve resections, chest wall involvement, and extensive adhesions whereas many surgeons would otherwise opt (wisely) for thoracotomy in these situations. In addition, surgeons who are unhappy about using 2-dimensional imaging with lack of depth perception and straight non-articulating instruments for the oncologic and vascular needs of lung cancer surgery may become convinced that a robotic portal lobectomy is indeed just a minimally invasive way of doing this routine operation through a thoracotomy. It is simply surgery through a virtual thoracotomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Horn L, Johnson DH., Evarts A. Graham and the first pneumonectomy for lung cancer. J Clin Oncol 2008;26:3268-75. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Sakurai H, Asamura H. Sublobar resection for early-stage lung cancer. Transl Lung Cancer Res 2014;3:164-72. [PubMed]
- Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 1993;56:1285-9. [Crossref] [PubMed]
- Jaklitsch MT, DeCamp MM Jr, Liptay MJ, et al. Video-assisted thoracic surgery in the elderly. A review of 307 cases. Chest 1996;110:751-8. [Crossref] [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644-6. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Abbas AE. New nomenclature for robotic-assisted thoracic surgery also gets rid of RATS. J Thorac Cardiovasc Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Cerfolio R, Louie BE, Farivar AS, et al. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J Thorac Cardiovasc Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Chen X, Yang S, Guo W, et al. Robotic-assisted right middle lobectomy. AME Med J 2017;2:8. [Crossref]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Shen J. Prof. Abbas E. Abbas: a robotic thoracic practice can provide both clinical and financial benefits for an academic institution. J Thorac Dis 2017;9:E573-5. [Crossref] [PubMed]


