Adjuvant treatment of non-small cell lung cancer: focus on targeted therapy
Introduction
Optimal management of selected patients with completely resected stage II-IIIA non-small cell lung cancer (NSCLC) include adjuvant cisplatin-based chemotherapy (1). In this patient population, the addition of adjuvant chemotherapy following surgery has resulted into an absolute improvement in survival of 4–5% at 5 years (2,3). A small survival benefit might also be observed for stage IB patients, though it appears to be mainly confined to high-risk patients, namely those with large tumors (T ≥4 cm) (4). Cisplatin-based doublets including vinorelbine, gemcitabine, docetaxel and pemetrexed (for non-squamous histology only) seem to be equally effective adjuvant treatments, while the addition of bevacizumab, an anti-VEGF monoclonal antibody, to platinum-based chemotherapy was not found to improve survival in a randomized phase 3 trial (5).
In the era of precision medicine, predictive biomarkers have revolutionized the therapeutic approach to advanced disease. The best described targetable alterations are sensitizing epidermal growth factor receptor (EGFR) mutations in exon 19 (ex19del) and exon 21 (L858R), which account for roughly 90% of all EGFR-mutated NSCLCs, and echinoderm microtubule-associated protein-like 4 (EML-4)-anaplastic lymphoma kinase (ALK) rearrangements (6,7). Taken together, EGFR mutations and ALK rearrangements can be detected in up to 20% of advanced NSCLCs, and are preferentially found in non-squamous histology and/or young patients with never/light smoking history. Importantly, selective tyrosine kinase inhibitors (TKIs) have been developed for the treatment of NSCLCs that harbor these targetable gene alterations, namely gefitinib, erlotinib, and afatinib that target the tyrosine kinase domain of EGFR, and crizotinib that inhibits the tyrosine kinase domain of ALK fusion protein. In clinical trials, EGFR- and ALK-TKIs have been shown to improve progression-free survival (PFS) and response rates over platinum-based chemotherapy in biologically selected patients with EGFR-mutated and ALK-positive advanced NSCLC, respectively, thus emerging as a standard first-line option in this setting (6,8,9).
On this basis, it is reasonable to consider the use of targeted therapy also as adjuvant treatment of patients with resected tumors that harbor EGFR mutations or ALK rearrangements. The present review will briefly discuss the present and future of targeted therapy for patients with completely resected NSCLC.
Rationale for adjuvant targeted therapy
At the present time there is no evidence suggesting a worse prognosis for EGFR-mutated NSCLC in early stage disease. A recent meta-analysis of 14 studies involving 2,652 cases of completely resected NSCLCs (EGFR-mutated =1,033, 39%) found that disease-free survival (DFS) of EGFR-mutated patients was not significantly different from that of EGFR wild type patients (HR 0.87; 95% CI 0.65–1.16) (10). Although this study did not address the impact of adjuvant chemotherapy in the analyzed population, it still provides compelling results on the fact that EGFR mutation is not prognostic in completely resected NSCLC. Similarly, retrospective studies exploring the impact of ALK rearrangements in early stage NSCLC suggested that ALK status lacks a prognostic role, as no significant difference in DFS was found between ALK-rearranged and ALK-negative patients (11,12). Nevertheless, EGFR mutations and ALK rearrangements are both highly predictive of response to selected targeted therapy in advanced NSCLC.
Against this background, a number of retrospective studies have suggested that targeted therapy has a role as adjuvant treatment of completely resected NSCLCs with EGFR-mutated NSCLC. The first study to set the stage for adjuvant EGFR-TKIs was published from Janjigian and colleagues who reported their experience on 167 patients with EGFR-mutated (ex19del and L858R) completely resected stage I-III NSCLC (13). Peri-operative (either adjuvant or neoadjuvant) gefitinib or erlotinib were administered in 56 patients (33%), the majority of whom (n=30, 54%) had stage I disease. In a multivariate analysis adjusted for sex, pathologic stage, type of surgery, and adjuvant cisplatin-based chemotherapy, patients treated peri-operatively with an EGFR-TKI had a 2-year DFS rate of 89% compared with 72% for patients who did not receive an EGFR-TKI (HR 0.53; 95% CI: 0.28–1.03; P=0.06). The 2-year overall survival (OS) was 96% versus 90%, respectively (HR 0.62; 95% CI: 0.26–1.51; P=0.296). Interestingly, an update analysis of 286 patients of whom 84 (29%) were treated with peri-operative gefitinib or erlotinib showed a significantly longer DFS in favor of adjuvant EGFR-TKI (HR 0.43; 95% CI: 0.26–0.72; P=0.001), with a non-significant trend for improved OS (HR 0.50; 95% CI: 0.23–1.08; P=0.076) (14). More recently, Lv and colleagues evaluated retrospectively 257 patients with completely resected stage I–IIIA NSCLC, of which 138 (53.7%) tested positive for an EGFR mutation (ex19del and L858R in 132 patients, 51.4%) (15). Among the EGFR-mutated population with ex19del and L858R they compared the patients who received an adjuvant EGFR-TKI (n=30) versus those who did not (n=102), and found a significantly higher DFS in the EGFR-TKI-treated group (P=0.033). Adjuvant EGFR-TKIs were not associated with improved OS (P=0.258), although the recipients had better 3-year OS (92.5% vs. 81%). However, approximately half of the patients treated with an adjuvant EGFR-TKI had stage IIIA disease, meaning that the benefit in DFS could have been largely driven by this high risk population. At the present time, no retrospective studies on the potential impact of an ALK-TKI on the outcome of ALK-positive early stage NSCLC have been reported.
Prospective trials of adjuvant targeted therapy
As shown in Table 1, a few prospective trials have investigated an EGFR-TKI as adjuvant treatment for patients with completely resected NSCLC. Unfortunately, most of these trials present with caveats in the study design and conduction. Among these, NCIC CTG BR.19, in which patients with completely resected stage IB-IIIA NSCLC treated or not with adjuvant platinum-based chemotherapy were randomized to either gefitinib or placebo for 2 years (16). Unfortunately, this trial was prematurely closed after randomizing only 503 of the 1,242 patients that were initially planned. This was the consequence of the negative outcomes observed in other phase 3 studies evaluating the role of gefitinib in either locally advanced disease or second-line setting (22,23). Therefore, the results of this study are totally uninformative. More recently, the results of the RADIANT trial, a phase 3 randomized, placebo-controlled trial of adjuvant erlotinib versus placebo for patients with stages IB–IIIA were published (17). In this study, 973 patients selected based on EGFR positivity by immunohistochemistry and/or fluorescence in situ hybridization were randomized in a 2:1 ratio to either erlotinib or placebo for 2 years, the primary end-point being DFS. Fifty-one percent of patients had stage IB disease, and approximately 52.9% of enrolled patients received adjuvant chemotherapy. The trial reported that DFS was not significantly different between the two arms of treatment [median 50.5 months for erlotinib and 48.2 months for placebo (HR =0.90; 95% CI: 0.74–1.10; P=0.324)], while OS data were immature at a median follow-up of 47 months. Interestingly, a trend in favor of erlotinib was evident in the subgroup of patients with an EGFR mutation (ex19del and L858R) (n=161, 16.5%) [median 46.4 months for erlotinib and 28.5 months for placebo (HR =0.61; 95% CI: 0.38–0.98)]. However, this finding should be interpreted with caution owing to the fact that the EGFR mutant patients assigned to the placebo arm underperformed when compared to the overall placebo-treated population, likely reflecting an imbalance in terms of patients characteristics (significantly more stage IIIA patients in the EGFR-mutated group treated with placebo) (17). More recent trials had the advantage to focus exclusively on the population of patients with an EGFR mutation, with promising results in terms of clinical outcome (18-20). Unfortunately, they were either uncontrolled studies (18) or randomized phase II studies (19,20) not powered enough in order to lead to conclusive results that could change clinical practice. Nevertheless, their results were consistent in suggesting that when patients are selected based on the presence of an EGFR mutation, adjuvant treatment with an EGFR-TKI with or without prior adjuvant chemotherapy is associated with a 2-year DFS ranging from 90% to 92.4%. Of note, these results are in line with the 2-year DFS of 89% reported by Janjigian and colleagues in their retrospective study (13). On this basis, a meta-analysis was conducted in order to evaluate the role of an adjuvant EGFR-TKI for EGFR-mutated completely resected NSCLCs (24). The results suggested a 9.5% absolute improvement in DFS at 3 years for EGFR-mutated NSCLCs treated with an adjuvant EGFR-TKI (HR =0.48), with a marginal, albeit statistically significant, benefit also in OS (HR =0.72). Although intriguing, the results of this meta-analysis are flawed by the fact that two of the five included studies were retrospective analyses, in which the patients who received an EGFR-TKI were not randomized to that therapy, thus potentially biasing the overall results (14,15).
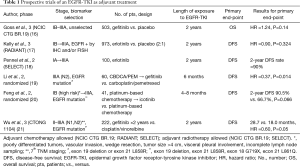
Full table
Recently, at ASCO 2017, Wu and colleagues reported the data coming from a randomized trial comparing gefitinib for 2 years versus four cycles of standard chemotherapy (cisplatin 75 mg/m2 day 1 + vinorelbine 25 mg/m2 days 1 and 8, every 3 weeks) in patients with completely resected stage II–IIIA NSCLC harboring an EGFR mutation (ex19del and L858R) (21). The primary end-point of the study, which was to demonstrate a 40% (HR =0.60) or greater improvement in DFS in favor of the gefitinib arm, was met. Two-hundred and twenty-two patients were randomized (111 in each arm), and median DFS was 28.7 months for gefitinib versus 18.0 months for chemotherapy (HR 0.60; 95% CI: 0.42–0.87; P=0.005), which translates into a 10.7 months improvement between the two treatments. Some points need to be addressed with regard to this study. First, it was conducted exclusively in a Chinese population. Secondly, it mainly focused on patients at high risk of recurrence, as documented by the fact that only individuals with pathologic lymph-node involvement N1-N2 were included. With this in mind, this study suggests that, when patients are properly selected based on the presence of an EGFR mutation, the benefit in favor of targeted therapy is clinically significant, and an EGFR-TKI may even replace adjuvant chemotherapy in this context. However, ongoing confirmatory studies will be crucial in order to assess the impact of an adjuvant EGFR-TKI in completely resected NSCLCs with an EGFR mutation.
Ongoing studies
Table 2 shows ongoing phase 3 randomized trials of targeted therapy as adjuvant treatment of completely resected NSCLC. In all of them, patients are selected for the appropriate targeted therapy based on the presence of either an EGFR mutation or ALK rearrangement. Importantly, these studies will provide an answer to most of the pending issues. First of all, should an EGFR-TKI be used sequentially after platinum-based chemotherapy (NCT02125240, NCT01996098, and NCT02193282/NCT02201992)? Rather, similarly to what has been suggested by Wu and colleagues, can an EGFR-TKI replace adjuvant chemotherapy as stand-alone treatment (WJOG6401L and NCT024488797)? Furthermore, what is the role for an EGFR-TKI given concomitantly with adjuvant chemotherapy (NCT02518802)? Finally, what is the optimal duration of adjuvant EGFR-TKI (NCT01996098)? With regard to the last issue, virtually all ongoing trials, in the absence of recurrence and/or unacceptable toxicity, anticipate that treatment with an EGFR-TKI should be continued for 2 years. However, such duration is to be balanced against the potential harm of treatment. In the RADIANT trial, in which erlotinib was also designed to be given for 24 months, the median duration of treatment was only 12 months for erlotinib versus 22 months for placebo, suggesting intolerance. In addition, as expected, the incidence of any grade adverse events was significantly higher with erlotinib versus placebo, the most common grade ≥3 adverse events being rash (22.3% vs. 0.3%) and diarrhea (6.2% vs. 0.3%) (17). On the other hand, a shorter duration of treatment may be still beneficial with the potential advantage of antagonizing the onset of biological resistance to treatment. According to this hypothesis, it has been demonstrated that patients who develop recurrence after stopping adjuvant EGFR-TKI are usually sensitive to re-treatment, while those who recur while on adjuvant EGFR-TKI are more likely to bear the exon 20 T790M mutation, which is associated with resistance to either first or second-generation EGFR-TKIs (gefitinib, erlotinib or afatinib) (25). With regard to this, the third-generation EGFR-TKI osimertinib has been demonstrated superior to gefitinib or erlotinib in first-line EGFR-mutated advanced NSCLCs (26). Similarly, the use of adjuvant osimertinib might prove beneficial, and the International study “ADAURA” is ongoing in order to compare osimertinib versus observation after standard adjuvant chemotherapy (NCT02511106).
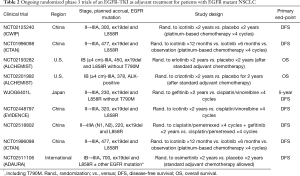
Full table
Conclusions and future directions
The aim of adjuvant treatment is to eradicate minimal residual disease. One of the concerns with adjuvant targeted therapy for completely resected NSCLCs is that it can only suppress rather than eliminate the growth of residual disease. If this is the case, patients who do not receive adjuvant targeted therapy may derive equal benefit by receiving it at recurrence. For this reason, the use of adjuvant targeted therapy could be justified only in presence of an OS benefit.
Another critical issue is how to select the patients who may need adjuvant targeted therapy. Measurement of residual disease by circulating tumor cells and/or DNA could help identify the high-risk population. However, for these patients it should be clarified whether targeted therapy should be used sequentially after platinum-based chemotherapy or as stand-alone treatment; in addition doubts may exist on the optimal duration of targeted therapy in patients for whom residual disease after surgery is documented.
Finally, as we move towards a better understanding of the mechanisms that underlie resistance to targeted therapy in advanced NSCLC, clinical research should rapidly apply the new knowledge to clinical trials of adjuvant treatment. Therefore, better understanding of the biology at recurrence and novel testing strategies for residual disease are crucial in order to help select those patients who could benefit the most from adjuvant targeted therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Crinò L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v103-15. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015.CD011430. [PubMed]
- Bradbury P, Sivajohanathan D, Chan A, et al. Postoperative Adjuvant Systemic Therapy in Completely Resected Non-Small-Cell Lung Cancer: A Systematic Review. Clin Lung Cancer 2017;18:259-73.e8. [Crossref] [PubMed]
- Wakelee HA, Dahlberg SE, Keller SM, et al. E1505: adjuvant chemotherapy +/- bevacizumab for early stage NSCLC — Outcomes based on chemotherapy subsets. J Clin Oncol 2016;34:abstr 8507.
- Metro G, Crinò L. Advances on EGFR mutation for lung cancer. Transl Lung Cancer Res 2012;1:5-13. [PubMed]
- Metro G, Tazza M, Matocci R, et al. Optimal management of ALK-positive NSCLC progressing on crizotinib. Lung Cancer 2017;106:58-66. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Liang W, He Q, Wang W, et al. The impact of EGFR mutations on the prognosis of resected non-small cell lung cancer: a meta-analysis of literature. Ann Oncol 2017;28:abstr ii20-3.
- Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. [Crossref] [PubMed]
- Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 2014;84:121-6. [Crossref] [PubMed]
- Janjigian YY, Park BJ, Zakowski MF, et al. Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol 2011;6:569-75. [Crossref] [PubMed]
- D'Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012;7:1815-22. [Crossref] [PubMed]
- Lv C, An C, Feng Q, et al. A Retrospective Study of Stage I to IIIa Lung Adenocarcinoma After Resection: What Is the Optimal Adjuvant Modality for Patients With an EGFR Mutation? Clin Lung Cancer 2015;16:e173-81. [Crossref] [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol 2014;32:abstr 7514.
- Li N, Ou W, Ye X, et al. Pemetrexed-carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA-N2 non-small cell lung cancer harbouring EGFR mutations: a randomized, phase II study. Ann Surg Oncol 2014;21:2091-6. [Crossref] [PubMed]
- Feng S, Wang Y, Cai K, et al. Randomized Adjuvant Chemotherapy of EGFR-Mutated Non-Small Cell Lung Cancer Patients with or without Icotinib Consolidation Therapy. PLoS One 2015;10:e0140794. [Crossref] [PubMed]
- Wu YL, Zhong W, Wang Q, et al. Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-small-cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): A randomized, Phase III trial (CTONG 1104). J Clin Oncol 2017;35:abstr 8500.
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Huang Q, Li J, Sun Y, et al. Efficacy of EGFR Tyrosine Kinase Inhibitors in the Adjuvant Treatment for Operable Non-small Cell Lung Cancer by a Meta-Analysis. Chest 2016;149:1384-92. [Crossref] [PubMed]
- Oxnard GR, Janjigian YY, Arcila ME, et al. Maintained sensitivity to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer recurring after adjuvant erlotinib or gefitinib. Clin Cancer Res 2011;17:6322-8. [Crossref] [PubMed]
- Tagrisso significantly improves progression-free survival in the Phase III FLAURA trial for lung cancer. Available online: http://www.nasdaq.com/press-release/tagrisso-osimertinib-significantly-improves-progression-free-survival-in-the-phase-iii-flaura-tria-20170727-00585


