Percutaneous tracheostomy: a comprehensive review
Introduction
Tracheostomy is one of the oldest procedures known to mankind. Ancient Egyptians portrayed tracheostomy on their paintings before 3500 BC and Alexander the great was reported in 1000 BC to have saved the life of a soldier by making an incision in his trachea with the tip of his sword (1). While one of the first documented case of successful tracheostomy was performed in 1546 by the Italian surgeon Antonio Musa Brassavola, it was not till 1909 when the first detail description of surgical tracheostomy (ST) was reported (2) and tracheostomy became a well-defined procedure with its own set of technique, indications and contraindications (3).
Tracheostomy methods
Tracheostomy can be done either surgically or percutaneously
In open surgical technique, a 2–3 cm long incision is made in the anterior neck midway between the cricoid cartilage and the sternal notch, the skin and platysma are dissected (4-6). Strap muscles are then retracted (4) laterally to expose the thyroid isthmus, which is then mobilized or divided. After adequate hemostasis is achieved, a cricoid hook or lateral stay sutures are used as needed to expose the trachea and then a small opening is made on the trachea. Often a Bjork flap is created, where part of a tracheal cartilage is incised, folded and sutured to maintain patency of the stoma. The tracheostomy tube is then inserted through this stoma.
Compared to surgical technique, the percutaneous dilational tracheostomy (PDT) uses a modified Seldinger technique (7,8) where the trachea is accessed with a needle and then a guidewire is inserted. The tracheostomy tube is introduced over the guidewire after dilation. In this review, we will focus primarily on the percutaneous technique.
Since its introduction in 1985 (9), various modifications in percutaneous dilational techniques have been introduced and are briefly discussed.
Initially, the serial dilator technique was introduced where sequentially larger dilators were used to create a stoma (9). Later, instead of serial dilators, a single curved tapering dilator was introduced to dilate the stoma in one single step, which is now most commonly used. In the US, there are two commercially available kits that use a single tapering dilator (Ciaglia Blue Rhino; Cook Medical Inc., Bloomington, Indiana, and Portex ULTRAperc Single Stage Dilator Technique Kit, Smiths Medical Dublin, Ohio). The use of a single dilator has minimized complications associated with serial dilation that were seen at the outset. A technique introduced in 1997 uses a specially designed Griggs forceps to dilate the stoma (10) that is introduced over a guidewire and then extended to dilate the stoma. Another technique was described by Fantoni et al., where after introduction of a guidewire in the anterior tracheal wall, the dilator and the tracheostomy tube are pulled through the larynx in a retrograde fashion (11). In percu-twist technique, a screw like dilator is slowly introduced over a guidewire by rotating it clockwise to dilate the stoma (12). A balloon dilation technique was introduced in the US where instead of a tapering dilator, a balloon was introduced over the guidewire to dilate the stoma (13). Once the stoma is dilated, the tracheostomy tube that was loaded proximal to the balloon is then passed over the guidewire.
In theory, except in Griggs, Fantoni’s and the balloon dilation techniques, all other methods require pressure over the anterior wall during dilation risking a possible posterior tracheal wall injury. However, experience and care with the single dilator technique has made it most popular with minimal risk of complications.
Technique of single dilator percutaneous tracheostomy (PT)
PT is mostly performed in the intensive care unit (ICU) where the patient is already intubated and sedated. However, it can be done anywhere. We prefer to have an airway in place, so the patient can be ventilated during the procedure. In addition to the operator, other personnel required for the procedure include a bronchoscopist and bronchoscopy technician, respiratory therapist to retract and hold the endotracheal tube (ETT) and a nurse to administer medications and monitor patient vitals. Adequate analgesia and sedation is required during the procedure. The anterior neck area is infiltrated with a combination of lidocaine and epinephrine to achieve vasoconstriction along with local anesthesia. Commonly used medications include fentanyl, midazolam, propofol, morphine and a short acting paralytic. However, PT has been reported in awake patient with only local anesthesia (14).
After obtaining informed consent, the patient is placed in a supine position. A rolled up towel is placed under the shoulder to extend the neck, so, the trachea can be easily palpated and the cricoid cartilage is pulled superiorly. This enhances the distance between the sternal notch and the cricoid cartilage allowing easier access to trachea. The tracheal rings are palpated to identify the sternal notch and the cricoid cartilage. Once the landmarks (sternal notch, tracheal rings and cricoid cartilage) are identified, the anterior neck area is cleaned and the surface is covered with sterile drape. If the cricoid cartilage or tracheal rings cannot be palpated, we prefer to avoid the percutaneous technique.
After adequate sedation is achieved, a short-acting paralytic is administered. A bronchoscope is then inserted through the ETT and a quick airway examination is done. The ETT is then slowly retracted under bronchoscopic guidance to the level of the cricoid cartilage after the cuff is deflated. We continue to retract the ETT until the cricothyroid membrane is visualized with the bronchoscope to identify the cricoid cartilage. The ETT tip is positioned at the level of the cricoid cartilage and the cuff of the ETT is then re-inflated. A 1.5-cm vertical incision is made in midline (Figure 1). With help of curved Kelly forceps and blunt dissection the trachea is slowly approached. The 1st, 2nd tracheal rings and the cricoid cartilage are identified with indentation from outside and by visualization through the bronchoscope.
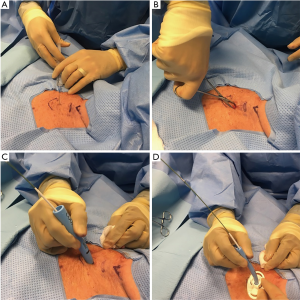
A needle is then inserted in midline through the interspace between the 1st and 2nd or 2nd and 3rd tracheal rings. Using Seldinger technique, a guidewire is inserted through the needle and the needle is removed. A 14F punch dilator is then inserted over the guidewire and next a curved, tapering dilator is inserted to dilate the stoma. Once the stoma is adequately dilated, tracheostomy tube is loaded onto a straight dilator and then inserted over the guidewire into the trachea. Bronchoscope is then removed from the ETT and inserted through the tracheostomy tube to confirm placement by visualizing the trachea or the carina. The distal end of the tracheostomy tube should be at least 1 inch or more proximal to the main carina.
Tracheostomy tube is then attached to ventilator circuit and a second mode of confirmation is made after an appropriate tidal volume return is detected on the ventilator. The tracheostomy tube is then secured with Velcro tracheostomy ties or sutures.
Indications
Indications for PT are similar to ST. In patients in the medical ICU, the common indication for tracheostomy is respiratory failure and the need for continued mechanical ventilation (15,16). About 5–10% of the ICU population require prolonged mechanical ventilation (17).
Upper airway obstruction is another indication for tracheostomy as in trauma, angioedema, malignancy and obstructive sleep apnea.
Often tracheostomy may need to be done emergently to secure the airway. A tracheostomy is often done to assist with clearance of profuse secretions. ST is preferred when there is malignancy involving the neck or upper airways.
Contraindications
Infection on neck wall and unstable patient are contraindications for tracheostomy. Historically, relative contraindications for the procedure were obesity, bleeding diatheses, limited neck mobility (e.g., trauma patients or when neck cannot be extended), distorted anatomy or prior neck surgery (including prior tracheostomy), and high ventilator support. However, with more experience, newer data suggest that PT can be done safely in these patients previously thought to be at higher risk. The current literature for these relative contraindications is reviewed.
Obesity
In a cohort of 474 adults with 73 obese patients with body mass index (BMI) ≥27.5 kg/m2, four different percutaneous techniques were studied. The overall complication rate was 43.8% in the obese group compared to 18.2% in the control group (P<0.001) and obesity was associated with 4.9-fold increased risk for serious complications (18). Another prospective study of PT in 500 ICU patients found that, obese patients with a BMI of 30 kg/m2 or higher experienced a significantly greater complication rate compared to patients with a BMI of less than 30 kg/m2 (15% vs. 8%, P<0.05) (19). However, a similar association was also observed in another report of open ST performed in 89 morbidly obese (BMI of ≥40 kg/m2) patients from a sample of 427 critically ill patients. Morbid obesity was independently associated with an increased risk (odds ratio 4.4) of tracheostomy-related complications (20).
On the other hand, multiple studies showed the safety of PT in obese patients without a higher complication rate (21,22). A retrospective study looking at 143 patients with BMI >35 kg/m2 who underwent either PT or open ST showed no significant difference in complication rates including malpositioning of tracheostomy tube, loss of airway, or bleeding (21). Bedside PT was safely performed in thirteen obese ICU patients (BMI ≥27 kg/m2) who required tracheostomy for failure to wean and procedural complications were limited to paratracheal tracheostomy tube placement in one patient, with immediate identification and appropriate correction (22). We believe that PT can be done safely in obese population with additional skills and experience.
Coagulopathy
PT was safely performed in a retrospective study of 42 patients with severe thrombocytopenia [platelet count 26.4±11.6×109 (mean ± SD) cells/L]. Twenty-two patients also had partial thromboplastin time (PTT) >40 sec and elevated international normalized ratio (INR) >1.5. Forty patients received platelet transfusion before the procedure and only two developed major post procedural bleeding that required suturing (23).
A retrospective analysis of 177 patients who underwent PT found no significant difference in bleeding in 16 (9.0%) coagulopathic (INR ≥1.7 or platelet count ≤50 k) patients compared to those without coagulopathy (24). Available data suggest that the incidence of bleeding is low in patients with coagulopathy or thrombocytopenia undergoing PT (25).
We prefer a platelet count of at least 50 k and INR <1.8 prior to performing PT. Heparin drip should be held for at least 4 hours prior to the PT and can be restarted 3 hours post-procedure. Safety of PT on anti-platelet therapy has been demonstrated (26). In patients where clopidogrel cannot be held for 3–5 days (e.g., recent drug eluting stent) we perform PT with appropriate informed consent.
Unextended neck
Traditionally neck is extended during PT. However, PT can be performed safely without neck extension such as in trauma or patients with cervical spine surgery, provided there is palpable trachea and suitable neck anatomy
In a series of 88 consecutive trauma patients undergoing PT, the success rate was 100% for the cleared group compared with 96% for the non-cleared group. The complication rate or success rate were not significantly different between the two groups and there was no spinal cord injury from the tracheostomy (27).
A study of 275 adult trauma patients with and without spinal cord injury reported no deaths or complications, wound infection or nonunion who underwent ST after anterior cervical spine fixation (ACSF) (28).
In a randomized study of 16 patients undergoing ST or ultrasound guided PT after ACSF, no perioperative complications were reported (29). One patient in each group had prolonged bleeding that stopped spontaneously. Twenty-five percent in the ST group developed infection and the procedure length was significantly shorter in the PT group (21 vs. 8 min, P30).
Repeat tracheostomy
Bedside PT can be successfully performed in critically ill patients with a history of previous tracheostomy and no significant periprocedural complications, and surgical revision is not necessary (31). Repeat PT is not difficult in patients with previous ST, where the previous stoma or the defect is usually easily palpable. There is a concern that the wound healing may be impaired or there may be difficulty, if new stoma is created on the previous scar tissue, however, this is not substantiated. In our practice, we prefer to go through the previous scar tissue or at the site of the old stoma.
High ventilator settings
A prospective study of 88 patients with high positive end expiratory pressure (PEEP) (16.6±4 cmH2O) compared to 115 patients with low PEEP (7.6±2.2 cmH2O) showed that bronchoscopically guided PT did not significantly decrease oxygenation in either group. Patients with average PaO2/FiO2 of 130 mmHg and an average PEEP of 17 cmH2O did not suffer any oxygenation deterioration (32).
Five patients with acute respiratory distress syndrome (ARDS) on high-frequency oscillatory ventilation had PDT safely performed without any significant hemodynamic or respiratory changes (33).
In a retrospective analysis of 177 patients, PT was successfully performed in 14 (8.4%) patients with high ventilator demand (HVD) (PEEP ≥10 cmH2O or FiO2 ≥70%) compared to non-HVD group without any significant differences in complications like hypoxia (7% vs. 6%), airway loss (14% vs. 4%), air-leak (7% vs. 3%) or para-tracheal placement (0% vs. 1%). However, hypotension (36% vs. 8%; P=0.007) and extra-long tracheostomy tube placement (43% vs. 12%; P=0.006) were significantly higher in the HVD group indicating that there is an interaction of obesity and HVD (24).
In our practice, we carefully evaluate patients on high FiO2 or high PEEP prior to PT to assess the overall risk. The shorter duration of PT and the use of bronchoscope through the ETT may actually minimize the loss of PEEP during the procedure. In this population, we usually retract the ETT when the blunt dissection is completed and the tracheal rings are identified by palpation, to minimize loss of PEEP or hypoxia.
Hypotension
Hypotension is a relative contraindication, especially if a patient is on vasopressor support. We generally avoid PT on a patient with multiple pressors or high dose of single pressor (e.g., more than 0.05 mcg/kg/min of norepinephrine or equivalent) at baseline. Dopamine can trigger arrhythmia. Of note, administration of sedation medications in preparation for tracheostomy can also lead to transient hypotension.
Timing of tracheostomy
There is no consensus on the optimal timing of tracheostomy (34) and variation across institutions can be substantial (35). In an international multicenter study involving 412 medical-surgical ICU patients, tracheostomy was performed at a median of 11 days (36) after intubation. The decision is mostly influenced by the patient’s overall clinical condition, prognosis and tolerance to wean.
In a prospectively randomized study of 120 critically ill medical patients, early PT within 48 hours was associated with significantly lower rate of mortality, pneumonia, accidental extubations and shorter length of stay in the ICU and on mechanical ventilation compared with the prolonged intubation group (37).
While similar findings were reported in reducing the rate of ventilator-associated pneumonia (38), decreased ICU length of stay and number of days spent on mechanical ventilation (38-41); the incidence of pneumonia was not significantly different in other studies with a mix of both ST and PT (40,41).
A meta-analysis of nine randomized clinical trials with 2,072 participants showed that early tracheostomy had no significant influence on clinical outcomes (short-term mortality, long-term mortality, length of ICU stay, ventilator-associated pneumonia or duration of mechanical ventilation) compared to late tracheostomy or prolonged intubation (42). Although, two more recent analyses suggest superiority of early tracheostomy (43,44), the timing of tracheostomy is dependent mostly on individual and institutional practice.
Complications of tracheostomy
Bleeding is the most common early complication of tracheostomy and was the major cause of death in one study (45). Minor oozing during placement may be managed by local pressure, light packing with adrenaline or tranexamic acid-soaked gauze packs (46). In case of ongoing bleeding or oozing around the tracheostomy tube after placement, the stoma needs to be explored to locate a bleeding vessel which can then be either cauterized or sutured.
Tracheo-innominate artery fistula is an uncommon (<1%) but fatal complication after tracheostomy (47,48) and can occur after 3–4 weeks after tracheostomy regardless of whether it was ST or PT (48). Tracheo-innominate artery fistula can be prevented by placing the tracheostomy tube in between the first and the second or second and the third tracheal rings. The risk is higher when the tube placement is further lower (49). The treatment is immediate surgical intervention. Direct digital pressure on the anterior wall of the stoma can be a helpful temporary measurement.
Respiratory secretions and mucus plugs, often occlude the tracheostomy tube cannula (50) in up to 57% of patients (51). Obstruction of the tracheal cannula by hematoma and swollen posterior tracheal wall is also possible (52).
Subcutaneous emphysema can occur in 1.4% and pneumothorax in 0.8% of cases after tracheostomy (53). However, the highest rate of pneumothorax is reported to be 17% (54). Four deaths resulting from pneumothorax after PT were reported and chronic obstructive pulmonary disease (COPD) was identified as a risk factor (45). Possible mechanisms for subcutaneous emphysema are malposition of the tracheostomy tube inside the stoma or air leaking into the subcutaneous tissue during ventilation during a difficult procedure. Subcutaneous emphysema can also happen in obese patients during dissection of the anterior neck tissue. Posterior tracheal wall laceration is another potential factor for pneumothorax (53).
In a retrospective study of 1,130 consecutive ST, the incidence of tracheostomy tube displacement was 1.5% (46). Tube displacement can be life-threatening immediately following a tracheostomy procedure before the track matures. And the ETT can be inadvertently reinserted paratracheally into a false track. The risk is higher in obese patients where the skin to tracheal distance may be in excess of 2–4 cm, especially if not in expert hands. This can be minimized when skin flaps are sutured to the trachea to form a permanent stoma or stay sutures are used (50). Restricting first tracheostomy tube change after 5–7 days can also minimize paratracheal insertion. Use of a guiding catheter (Weinmann tracheostomy exchange set, COOK Medical Inc. Bloomington, Indiana, USA) during tracheostomy tube exchange and bronchoscopic guidance can minimize the risk of paratracheal insertion.
We routinely place extra-long tracheostomy tubes in obese patients, when skin to trachea distance is greater than 1cm or when the trachea is deep, to avoid malposition of the tracheostomy tube that can lead to cuff leak.
At our institution, loss of airway or cuff leak after PT was a common occurrence in the obese population during the first 50 cases. The number reduced significantly once we started to place extra-long tracheostomy tubes in patients with obesity, short neck and deep trachea (55).
Tracheal stenosis is narrowing of the tracheal lumen from fibrosis or granulation tissue formation after intubation or tracheostomy tube placement with an incidence as high as 85% (56). Only 3–12% are clinically important and require further intervention (48). As a rule, symptoms develop when the lumen is <50% of normal. Stomal stenosis may start as bacterial infection and chondritis leading to formation of granulation tissue while stenosis at the cuff level is due to pressure necrosis (48). A-form deformities can develop later when the tracheal cartilage is fractured during placement or if a Bjork flap was created during ST.
Rigid or flexible bronchoscopy with radial resection of the stenotic area with cautery knife, balloon dilation, cryotherapy, mitomycin application are possible treatment options before considering surgical resection (56).
Tracheoesophageal fistula (TEF) is very rare (57). In one review of 1,130 tracheostomies, the incidence of TEF was 0.08% and was universally fatal (46). Injury to the posterior tracheal wall during stoma creation, during intubation or erosion of the tracheostomy cuff through the posterior tracheal wall due to excessive cuff pressures (54) are potential risk factors for TEF.
Stomal infection can occur at any time after tracheostomy placement, as the tracheal secretions can easily contaminate the wound. In chronic tracheostomy, pressure of the tube flange or swivel adapter edge against the sternum and poor tracheostomy tube care can make it worse. There is evidence that stomal infection occurs less frequently in patients with PT (58). It is also possible that the majority of MICU patients who undergo PT are already on antibiotic.
Overall, evidence suggests that in critically ill patients, elective tracheostomy can be carried out with acceptable perioperative risk profile (59). Table 1 summarizes the potential complications of PT.

Full table
PT versus ST
A meta-analysis of 21 ST studies on 3,512 patients and 27 PT studies on 1,817 patients showed that PT is associated with higher perioperative complications and postoperative complication rates are higher with ST (60).
Another meta-analysis that pooled data from five studies (236 patients) showed that PT was associated with shorter operative time, less perioperative bleeding and lower postoperative incidence of stomal infection compared to ST. There was no difference in overall operative complication rates or mortality between ST and PT (61).
Seventeen RCTs involving 1,212 patients in a meta-analysis comparing PT to ST found that PT has lower risk of wound infection, bleeding and mortality when compared with ST (62).
Overall, PT in well-trained hands is a procedure with excellent safety records in variety of patients with many of the aforementioned contraindications (63,64). Bronchoscopy guided PT is a safe procedure when performed by a team of experienced operators in a controlled environment with less than 3% risk of significant complications (52).
In a prospective randomized study comparing PT and ST in critically ill patients, the cost of PT was found to be significantly lower (US$ 1,569±157 vs. US$ 3,172±114; P<0.0001), the duration of PT was significantly shorter (20 vs. 42 mins; P<0.0001) and there was no significant difference in the number of days intubated prior to tracheostomy or overall ICU and hospital length of stay compared to ST (65).
A meta-analysis of 22 studies comparing PT and ST found no significant differences in mortality and intraoperative or post-operative bleeding risk. There was a significantly lower rate of infection (pooled odds ratio 0.20 P≤0.0001) and shorter procedure time (pooled OR −1.7 to 0.7, P≤0.001) with PT compared to ST (66).
Another study of 61 ICU patients who underwent bronchoscopy guided PT reported one procedure-related death due to arrhythmia (67). There was a 50% reduction in cost with PT compared to operative tracheostomy (67).
Role of ultrasound in PT
The use of ultrasound in PT can be advantageous especially in select patient groups, such as those who are morbidly obese or have difficult neck anatomy (68-70). Bedside ultrasound screening allows for easy identification of pretracheal vascular structures that might pose a hemorrhage risk during PT (71). In addition, it can also provide an estimate of skin to trachea distance, especially in obese population.
Portable ultrasound before PT to identify the tracheal rings, thyroid isthmus, neighboring blood vessels may help provide a safe location for needle insertion (72). A randomized prospective study of 47 patients found that real-time ultrasound guidance can significantly improve the rate of first-pass puncture and puncture accuracy (73).
However, posterior tracheal wall cannot be visualized with ultrasound due to the tissue to air interface and posterior wall damage during dilatational PT cannot be assessed with ultrasound. Moreover, routine use of real time ultrasound can be cumbersome especially in the small operating field of tracheostomy.
Role of bronchoscopy
Procedure duration was shorter when bronchoscopy is used (16 vs. 45 mins, P<0.0001) in a prospective study of 48 trauma patients in the OR (74).
However, safe tracheostomy tube placement was possible in the absence of bronchoscopic guidance by ensuring free mobility of the guide wire at each step of PT using dilating forceps (Griggs) in a retrospective study of 98 patients (75). The mean operating time was 3.05 minutes, and there were no deaths or life threatening complications.
There was no significant difference in complication rate or procedural duration when ultrasound guided PDT is compared to bronchoscopy guided PDT in a prospective randomized trial of 118 patients (76).
Suture or no suture
Tracheostomy tube displacement may lead to potentially serious complications and loss of airway. Fixation of the tracheostomy tube to the skin with a suture can potentially minimize accidental decannulation in PT (77). However, one needs to remember that skin is very mobile, especially in obese patients. The distal end of a regular tracheostomy tube may be dislodged inside the tracheostomy stoma leading to cuff leak or subcutaneous emphysema, while the tracheostomy flange may still be sutured to the skin. In one large series of 1,175 tracheotomies, accidental decannulation was not found to be associated with suture placement. Moreover, accidental decannulation occurred in patients while the suture was still in place (78). A tracheostomy tie is the most important factor in holding the tracheostomy tube in place. The same study found that use of outer flange security sutures to anchor the tracheostomy tube was associated with lower incidence of postoperative bleeding (78).
In our practice, we do not place any sutures during PT, and use a Velcro tracheostomy tie. The tracheostomy tie is applied in such way, that it is tight and snug but still permits a finger breadth of slack around the neck. Prior to implementing a no suture policy, it is important to educate and train the nursing stuff and other caretakers to prevent any accidental decannulation.
The application of stay suture (placement of sutures between the anterior tracheal wall and the skin to hasten the formation of a mature stoma) was evaluated in 104 patients prospectively and compared to 101 conventional tracheostomy patients. The most common complication in each group was postoperative stoma infection. Unexpected decannulation occurred in three patients in the conventional tracheostomy group, while none occurred in the stay suture group (79).
Training and competency
A learning curve has been identified for percutaneous dilational tracheostomies (80). Perioperative and late complication rates were significantly reduced after the first 20 patients. Practice on mannequins or animal model during initial training can improve the skills and minimize complications. A study of emergency medicine residents on cricothyroidotomy found that training on live anesthetized animals improve procedural competency and performance (81). The technique of PT can be acquired after 5–10 procedures; however, supervised experience with at least 20 procedures is needed to become familiar with common difficulties, potential complications and risks. In a multicenter study of tracheostomies, surgeons with fewest tracheostomies were found to have highest complication rates (78). We recommend that, the high risk cases or patients with relative contraindication be attempted only after experience with a total of 50–100 PTs.
Post tracheostomy care and decannulation
Special care is needed to manage tracheostomy after placement. The wound is maintained clean and dry. Primary operator is notified of any bleeding or cuff-leak in the immediate post-operative period, in case the tracheostomy stoma needs to be explored or the tracheostomy tube needs to be replaced. Most medical ICU patients are usually transferred to a ventilator weaning facility, where experienced physicians or respiratory therapists manage ventilator weaning and provide care of the tracheostomy.
A patient may be weaned off from a ventilator but may still need long term tracheostomy for secretion clearance, upper airway obstruction or neck surgery. The patient or the caretaker should be educated on how to clean and care for the tracheostomy tube and suctioning prior to discharging the patient. Disposable inner-cannula can be an alternative to regular cleaning. A heat and moisture exchange (HME) filter maintains moisture of the airways and prevent formation of mucus plug. Regular follow up with respiratory or speech therapist or other health care provider experienced in managing long term tracheostomy ensures proper maintenance. Although optimum interval is unknown, tracheostomy tube change by an experienced provider every 2–3 months is a good practice.
Decannulation
The process of decannulation depends on institutional protocol and practice. Once a patient is weaned from mechanical ventilation, has strong cough and is able to clear secretions; the process of decannulation is beguan. The tracheostomy tube is downsized to a smaller caliber tube to allow increased trans-laryngeal airflow and thus improve speech. At this point, patients may be able to speak by blocking the tracheostomy tube opening with a finger or with the help of a one-way speaking valve. Often, a fenestrated or cuff less tracheostomy tube is used to permit additional airflow.
Once the patient is able to tolerate the speaking valve, the next step is to cap the tracheostomy tube, which forces to inhale and exhale through the upper airways instead of the tracheostomy tube, essentially preparing the patient for decannulation. If the patient is able to tolerate capping of the tracheostomy tube for more than 48–72 hours, the tracheostomy tube can be removed. A bronchoscopic examination is indicated if the patient in unable to tolerate capping to rule out any stenosis or granulation tissue formation.
Conclusions
Tracheostomy is a common procedure. PT is safe, cost-effective and is performed by both surgeons and non-surgeons, while ST, as the name implies is performed primarily by surgeons. The complication profile for both techniques is comparable. With experience, PT can be performed in patient population previously thought to be of high risk. A large worldwide tracheostomy database will be able to provide an accurate information on the cost, risk and complications of various techniques of tracheostomy in different patient population.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Shaheen Islam served as a paid consultant for COOK Medical Inc. during 2013-2015; Dr. Ashraf Rashid has no conflicts of interest to declare.
References
- Frost EA. Tracing the Tracheostomy. Ann Otol Rhinol Laryngol 1976;85:618-24. [Crossref] [PubMed]
- Tracheotomy Jackson C. Laryngoscope 1909;9:285-90.
- Rajesh O, Meher R. Historical Review Of Tracheostomy. The Internet Journal of Otorhinolaryngology 2005;4.
- Cameron JL. Tracheostomy. In: Cameron JL, Cameron AM. editor. Current Surgical Therapy. 12th edition. Elsevier, 2016.
- Zollinger RM Jr, Ellison EC, Bitans M, et al. Zollinger’s atlas of surgical operations. New York (NY): McGraw-Hill, 2003.
- Cameron JL. Current surgical therapy. Philadelphia (PA): Mosby, 2008.
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol 1953;39:368-76. [Crossref] [PubMed]
- Pelausa EO. Percutaneous tracheostomy: ready or not? J Otolaryngol 1991;20:88-92. [PubMed]
- Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest 1985;87:715-9. [Crossref] [PubMed]
- Griggs WM, Worthley LI, Gilligan JE, et al. A simple percutaneous tracheostomy technique. Surg Gynecol Obstet 1990;170:543-5. [PubMed]
- Fantoni A, Ripamonti D. A non-derivative, non-surgical tracheostomy: the translaryngeal method. Intensive Care Med 1997;23:386-92. [Crossref] [PubMed]
- Frova G, Quintel M. A new simple method for percutaneous tracheostomy: controlled rotating dilation. A preliminary report. Intensive Care Med 2002;28:299-303. [Crossref] [PubMed]
- Zgoda MA, Berger R. Balloon-facilitated percutaneous dilational tracheostomy tube placement: preliminary report of a novel technique. Chest 2005;128:3688-90. [Crossref] [PubMed]
- Rashid A, Raj B, Stoddart A. Repeat percutaneous dilatational tracheostomy in an awake and unintubated patient. Acta Anaesthesiol Scand 2007;51:378-9. [Crossref] [PubMed]
- Plummer AL, Gracey DR. Consensus Conference on Artificial Airways in Patients Receiving Mechanical Ventilation. Chest 1989;96:178-80. [Crossref] [PubMed]
- MacIntyre NR. Evidence-Based Guidelines for Weaning and Discontinuing Ventilatory Support. Chest 2001;120:375S-95S. [Crossref] [PubMed]
- Cohen IL, Booth FV. Cost containment and mechanical ventilation in the United States. New Horiz 1994;2:283-90. [PubMed]
- Byhahn C, Lischke V, Meininger D, et al. Peri-operative complications during percutaneous tracheostomy in obese patients. Anaesthesia 2005;60:12-5. [Crossref] [PubMed]
- Kost KM. Endoscopic Percutaneous Dilatational Tracheotomy: A Prospective Evaluation of 500 Consecutive Cases. Laryngoscope 2005;115:1-30. [Crossref] [PubMed]
- El A, Solh AA, Jaafar W. comparative study of the complications of surgical tracheostomy in morbidly obese critically ill patients. Crit Care 2007;R3:11. [PubMed]
- Heyrosa MG, Melniczek DM, Rovito P, et al. Percutaneous Tracheostomy: A Safe Procedure in the Morbidly Obese. J Am Coll Surg 2006;202:618-22. [Crossref] [PubMed]
- Mansharamani NG, Koziel H, Garland R, et al. Safety of Bedside Percutaneous Dilatational Tracheostomy in Obese Patients in the ICU. Chest 2000;117:1426-9. [Crossref] [PubMed]
- Kluge S, Meyer A, Kühnelt P, et al. Percutaneous Tracheostomy Is Safe in Patients With Severe Thrombocytopenia. Chest 2004;126:547-51. [Crossref] [PubMed]
- Patel D, Devulapally K, Islam S. SAfety of percutaneous tracheostomy in patients with coagulopathy and high ventilatory demand. Chest 2009;136:50S-f-S.
- Al Dawood A, Haddad S, Arabi Y, et al. The safety of percutaneous tracheostomy in patients with coagulopathy or thrombocytopenia. Middle East J Anaesthesiol 2007;19:37-49. [PubMed]
- Abouzgheib W, Meena N, Jagtap P, et al. Percutaneous dilational tracheostomy in patients receiving antiplatelet therapy: is it safe? J Bronchology Interv Pulmonol 2013;20:322-5. [Crossref] [PubMed]
- Mayberry JC, Wu IC, Goldman RK, et al. Cervical spine clearance and neck extension during percutaneous tracheostomy in trauma patients. Crit Care Med 2000;28:3436-40. [Crossref] [PubMed]
- O’Keeffe T, Goldman RK, Mayberry JC, et al. Tracheostomy after anterior cervical spine fixation. J Trauma 2004;57:855-60. [Crossref] [PubMed]
- Sustic A, Krstulovic B, Eskinja N, et al. Surgical tracheostomy versus percutaneous dilational tracheostomy in patients with anterior cervical spine fixation: preliminary report. Spine (Phila Pa 1976) 2002;27:1942-5; discussion 5.
- Romero-Ganuza J, Gambarrutta C, Merlo-Gonzalez VE, et al. Complications of tracheostomy after anterior cervical spine fixation surgery. Am J Otolaryngol 2011;32:408-11. [Crossref] [PubMed]
- Meyer M, Critchlow J, Mansharamani N, et al. Repeat bedside percutaneous dilational tracheostomy is a safe procedure. Crit Care Med 2002;30:986-8. [Crossref] [PubMed]
- Beiderlinden M, Groeben H, Peters J. Safety of percutaneous dilational tracheostomy in patients ventilated with high positive end-expiratory pressure (PEEP). Intensive Care Med 2003;29:944-8. [Crossref] [PubMed]
- Shah S, Morgan P. Percutaneous dilation tracheostomy during high-frequency oscillatory ventilation. Crit Care Med 2002;30:1762-4. [Crossref] [PubMed]
- Gomes Silva BN, Andriolo RB, Saconato H, et al. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev 2012.CD007271. [PubMed]
- Mehta AB, Cooke CR, Wiener RS, et al. Hospital Variation in Early Tracheostomy in the United States: A Population-Based Study. Crit Care Med 2016;44:1506-14. [Crossref] [PubMed]
- Esteban A, Anzueto A, Alia I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000;161:1450-8. [Crossref] [PubMed]
- Rumbak MJ, Newton M, Truncale T, et al. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med 2004;32:1689-94. [Crossref] [PubMed]
- Möller MG, Slaikeu JD, Bonelli P, et al. Early tracheostomy versus late tracheostomy in the surgical intensive care unit. Am J Surg 2005;189:293-6. [Crossref] [PubMed]
- Arabi Y, Haddad S, Shirawi N, et al. Early tracheostomy in intensive care trauma patients improves resource utilization: a cohort study and literature review. Crit Care 2004;8:R347-52. [Crossref] [PubMed]
- Griffiths J, Barber VS, Morgan L, et al. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ 2005;330:1243. [Crossref] [PubMed]
- Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA 2010;303:1483-9. [Crossref] [PubMed]
- Huang H, Li Y, Ariani F, et al. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One 2014;9:e92981. [Crossref] [PubMed]
- Hosokawa K, Nishimura M, Egi M, et al. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care 2015;19:424. [Crossref] [PubMed]
- Andriolo BN, Andriolo RB, Saconato H, et al. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev 2015;1:CD007271. [PubMed]
- Simon M, Metschke M, Braune SA, et al. Püschel K, Death after percutaneous dilatational tracheostomy: a systematic review and analysis of risk factors. Critical Care 2013;17:R258. [Crossref] [PubMed]
- Goldenberg D, Ari EG, Golz A, et al. Tracheotomy complications: A retrospective study of 1130 cases. Otolaryngol Head Neck Surg 2000;123:495-500. [Crossref] [PubMed]
- Scalise P, Prunk SR, Healy D, et al. The Incidence of Tracheoarterial Fistula in Patients With Chronic Tracheostomy Tubes. Chest 2005;128:3906-9. [Crossref] [PubMed]
- Epstein SK. Late complications of tracheostomy. Respir Care 2005;50:542-9. [PubMed]
- Wood DE, Mathisen DJ. Late complications of tracheotomy. Clin Chest Med 1991;12:597-609. [PubMed]
- Mitchell RB, Hussey HM, Setzen G, et al. Clinical Consensus Statement: Tracheostomy Care. Otolaryngology -- Head and Neck Surgery 2013;148:6-20. [Crossref] [PubMed]
- Trottier SJ, Ritter S, Lakshmanan R, et al. Percutaneous Tracheostomy Tube Obstruction. Chest 2002;122:1377-81. [Crossref] [PubMed]
- Polderman KH, Spijkstra JJ, de Bree R, et al. Percutaneous Dilatational Tracheostomy in the ICU. Chest 2003;123:1595-602. [Crossref] [PubMed]
- Fikkers BG, van Veen JA, Kooloos JG, et al. Emphysema and Pneumothorax After Percutaneous Tracheostomy. Chest 2004;125:1805-14. [Crossref] [PubMed]
- Cipriano A, Mao ML, Hon HH, et al. An overview of complications associated with open and percutaneous tracheostomy procedures. Int J Crit Illn Inj Sci 2015;5:179-88. [Crossref] [PubMed]
- Chambers D, Cloyes R, Adam A, et al. Percutaneous Tracheostomy in Severe Obesity: Experience at a Tertiary Care Center. Chest 2013.144.
- Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: Report of 31 cases and review of the literature. BMC Pulmonary Medicine 2008;8:18. [Crossref] [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Holdgaard HO, Pedersen J, Jensen RH, et al. Percutaneous dilatational tracheostomy versus conventional surgical tracheostomy. A clinical randomised study. Acta Anaesthesiol Scand 1998;42:545-50. [Crossref] [PubMed]
- Stock MC, Woodward CG, Shapiro BA, et al. Perioperative complications of elective tracheostomy in critically ill patients. Critical Care Medicine 1986;14:861-3. [Crossref] [PubMed]
- Dulguerov P, Gysin C, Perneger TV, et al. Percutaneous or surgical tracheostomy: a meta-analysis. Crit Care Med 1999;27:1617-25. [Crossref] [PubMed]
- Freeman BD, Isabella K, Lin N, et al. A Meta-analysis of Prospective Trials Comparing Percutaneous and Surgical Tracheostomy in Critically Ill Patients. Chest 2000;118:1412-8. [Crossref] [PubMed]
- Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta‐analysis. Critical Care 2006;10:R55. [Crossref] [PubMed]
- Ernst A, Critchlow J. Percutaneous tracheostomy—special considerations. Clin Chest Med 2003;24:409-12. [Crossref] [PubMed]
- Al-Ansari MA, Hijazi MH. Clinical review: percutaneous dilatational tracheostomy. Critical Care 2006;10:202. [Crossref] [PubMed]
- Freeman BD, Isabella K, Cobb JP, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med 2001;29:926-30. [Crossref] [PubMed]
- Johnson-Obaseki S, Veljkovic A, Javidnia H. Complication rates of open surgical versus percutaneous tracheostomy in critically ill patients. Laryngoscope 2016;126:2459-67. [Crossref] [PubMed]
- Marelli D, Paul A, Manolidis S, et al. Endoscopic guided percutaneous tracheostomy:early results of a consecutive trial. J Trauma 1990;30:433-5. [Crossref] [PubMed]
- Rudas M. The role of ultrasound in percutaneous dilatational tracheostomy. Australas J Ultrasound Med 2012;15:143-8. [Crossref] [PubMed]
- Guinot PG, Zogheib E, Petiot S, et al. Ultrasound-guided percutaneous tracheostomy in critically ill obese patients. Crit Care 2012;16:R40.
- Rajajee V, Fletcher JJ, Rochlen LR, et al. Real-time ultrasound-guided percutaneous dilatational tracheostomy: a feasibility study. Crit Care 2011;15:R67. [Crossref]
- Flint AC, Midde R, Rao VA, et al. Ho ultrasound screening for pretracheal vascular structures may minimize the risks of percutaneous dilatational tracheostomy. Neurocrit Care 2009;11:372-6. [Crossref] [PubMed]
- Hatfield A, Bodenham A. Portable ultrasonic scanning of the anterior neck before percutaneous dilatational tracheostomy. Anaesthesia 1999;54:660-63. [Crossref] [PubMed]
- Rudas M, Seppelt I, Herkes R, et al. Traditional landmark versus ultrasound guided tracheal puncture during percutaneous dilatational tracheostomy in adult intensive care patients: a randomised controlled trial. Crit Care 2014;18:514. [Crossref] [PubMed]
- Barba CA, Angood PB, Kauder DR, et al. Bronchoscopic guidance makes percutaneous tracheostomy a safe, cost-effective, and easy-to-teach procedure. Surgery 1995;118:879-83. [Crossref] [PubMed]
- Maddali MM, Pratap M, Fahr J, et al. Percutaneous tracheostomy by guidewire dilating forceps technique: review of 98 patients. J Postgrad Med 2001;47:100-3. [PubMed]
- Gobatto AL, Besen BA, Tierno PF. Ultrasound-guided percutaneous dilational tracheostomy versus bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients (TRACHUS): a randomized noninferiority controlled trial. Intensive Care Med 2016;42:342. [Crossref] [PubMed]
- Beiderlinden M, Walz M, Sander A, et al. Karl Complications of bronchoscopically guided percutaneous dilational tracheostomy: beyond the learning curve. Intensive Care Med 2002;28:59-62. [Crossref] [PubMed]
- Halum SL, Ting JY, Plowman EK, et al. A multi-institutional analysis of tracheotomy complications. Laryngoscope 2012;122:38-45. [Crossref] [PubMed]
- Lee SH, Kim KH, Woo SH. The usefulness of the stay suture technique in tracheostomy. Laryngoscope 2015;125:1356-9. [Crossref] [PubMed]
- Massick DD, Powell DM, Price PD, et al. Quantification of the learning curve for percutaneous dilatational tracheotomy. Laryngoscope 2000;110:222-8. [Crossref] [PubMed]
- Custalow CB, Kline JA, Marx JA, et al. Emergency department resuscitative procedures: animal laboratory training improves procedural competency and speed. Acad Emerg Med 2002;9:575-86. [Crossref] [PubMed]


