Malignant pleural effusion and algorithm management
Background
Normally, 5 to 15 mL of pleural fluid present within the pleural space, formed by the parietal and visceral pleural membranes of hemithorax. About 5 to 10 liters of pleural fluid traverse the pleural space on a daily basis (1-7). Increased pleural fluid production due to increased vascular permeability or reduced reabsorption by lymphatics usually due to disrupted or occluded drainage channels. The presence of malignant pleural effusion (MPE) frequently indicates advanced disease and the primary goal in the management of MPE should be palliation of symptoms (palliative treatment). Bronchogenic carcinomas and breast cancers are the most common metastatic tumors to the pleura (40% and 25% respectively). Nevertheless, about 10% of all MPEs are due to primary cancers arising from the pleura with malignant mesothelioma as the predominant type (>90%) and cancer of unknown primary results in less than 10%. According to the International Association for the Study of Lung Cancer (IASLC), pleural dissemination of lung cancer, either by pleural effusion or pleural invasion without evidence of other metastatic foci, was revised to M1a categorizing it as stage IV disease (8).
Diagnosis of MPE
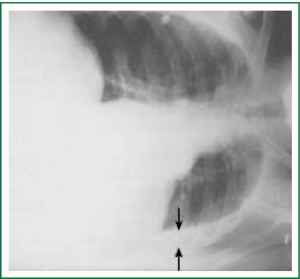
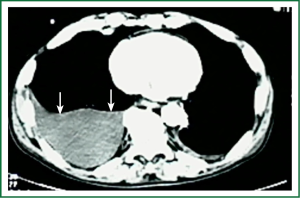
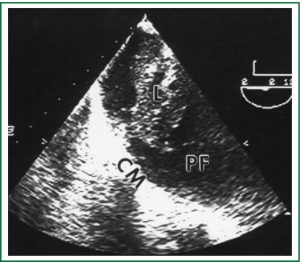
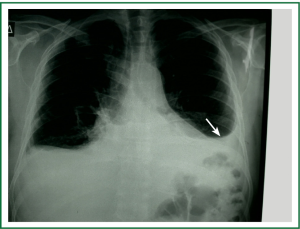
Diagnostic tests typically include a chest X-ray (effusions produce a meniscus sign along the lateral chest wall), chest computer tomography (CT) scan, MRI (especially to detect small effusions), thoracentesis, pleural fluid analysis and pleural biopsy. Lateral chest X-ray, lateral view or in case of small effusions a decubitus films can detect 100 cc of free flowing effusion. More than 90% of MPEs are exudatives. The appearance in half of them is hemorrhagic and in 11% is bloody in nature: Exudative properties after pleural fluid thoracentesis are commonly defined on the basis of the Light’s criteria (a pleural fluid-to-serum protein ratio of more than 0.5, a pleural fluid-to-serum lactic dehydrogenase ratio of more than 0.6, or a pleural fluid LDH level of 200 IU, pH of less than 7.30, pleural fluid glucose less than 60 mg/dL) (9,10). Cytological examinations of pleural fluid from thoracentesis have a diagnostic yield, ranging from 62% to 90% (11-16). Where the initial cytological evaluation was negative a closed biopsy could be combined with cytology. These diagnostic combinations increase the diagnosis as high as 80% (17).
In case of two negative consecutive, cytologic examinations medical thoracoscopy or Video-Assisted Thoracoscopic Surgery (VATS) are recommended. Diagnostic sensitivity for medical thoracoscopy and for VATS is greater than 90% with specificity of 100% while the operative mortality is less than 0.5% (18,19). Both procedures allow detailed investigation of pleural cavity and for visually detected lesions, directed pleural biopsies could be obtained. Epidermal Growth Factor Receptor (EGFR) mutations analysis of MPE biopsies in cases of non squamous non-small cell lung cancer (NSCLC) offers the benefit of EGFR-targeted therapies (20).
DNA methylation was detected in 59% of MPEs. But in none of the benign pleural effusions (21).
Standard examination of DNA methylation in MPEs may increase sensitivity of cytological evaluation (Figures 1-5).

Treatment
The main goals in the treatment of MPE are the removal of the effusion, the improvement in symptoms and the prevention of re-accumulation. Therapeutic thoracentesis and fluid aspiration should be the first medical procedure in the management of MPE which are useful in determining the effects on breathlessness. Thoracentesis has limited effect as a permanent therapeutic approach. More than 98% of MPE associated with lung cancer will relapse within 30 days from the first thoracentesis. Chemotherapy is effective in controlling the production only in responders NSCLC patients. On the other hand a successful management of MPE usually is observed in small cell lung cancer (SCLC) as many patients respond to chemotherapy.
Chemical pleurodesis
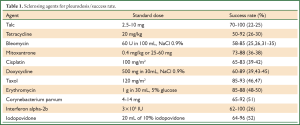
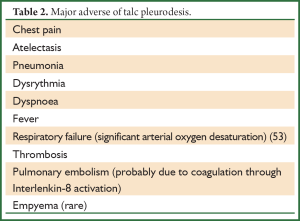
This procedure refers to application of sclerosant agents into the pleural cavity to achieve symphysis between the visceral and parietal pleura. The ideal sclerosant agent for chest catheter pleurodesis still remains controversial. A Cochrane review reported that pleurodesis with talc is superior when compared with other sclerotic agents (Table 1) or chest drainage alone to control an MPE or prevent the re-accumulation of fluid in the pleural cavity. The European Respiratory Society (ERS) and the American Thoracic Society (ATS) recommend not to remove more than 1.5l t of fluid on each occasion and to discontinue aspiration in case of signs of rapid pulmonary expansion (dyspnoea, chest pain, persistent cough). Fluid aspiration could be repeated at 2 hours intervals in case of persistent, severe dyspnoea due to large pleural effusions (Table 2). Experimental data demonstrated that talc stimulation of mesothelial cells have the capacity to promote intrapleural fibrosis. Talc also affects the angiogenic balance into pleural cavity and produces apoptosis of malignant mesothelial cells but not normal mesothelial cells.
Full Table
Full Table
Which talc and which policy?
Sterile asbestos-free graded (particle size >15 μm) talc is used for intrapleural administration in two ways. Either via thoracoscopy using an atomizer formed “talc poudrage”, either via an inter costal tube in the form of a suspension firmed “talc slurry”. The majority of similar studies show a slight superiority in “talc poudrage” but with no statistical significance as far as the control and the re-accumulation of the fluid.
Via the indwelling pleural catheter (IPC) prior to pleurodesis, 3 mg/kg lidocaine solution (1%) up to 250 mg should be administered in order to prevent local pain and discomfort. Also the use of sedation to prevent patient’s anxiety may be helpful.
Contra-indications
- Life expectancy <3 months;
- Failure of opposition of the pleural surfaces (trapped lung).
Trapped lung occurs: when lung expansion is restricted, either by visceral pleura tumour encasement or by endobronchial obstruction.
Successful pleurodesis often defined as no significant fluid re-accumulation in 30 days occurs in about 75% of pts in large clinical studies (54).
Tetracycline
There is some evidence that the sclerosant agent tetracycline stimulates mesothelial cells, releases growth factor, like activity for fibroplasts and this action may play an important role in inducing pleural fibrosis (55).
The reported success rate for control of MPEs with this agent ranged from 59% to 94% (56). The intravenous form used for intrapleural instillation is no longer commercially available in the United States and Greece.
Long-term IPC
IPC is preferred for patients with a trapped lung or failed pleurodesis or to provide apposition of the parietal and visceral pleural surfaces for subsequent pleurodesis. Three randomized trials investigating the efficacy between small- and large-bone chest tubes, all concluded that they were equivalent, but large-bone tubes are associated with significant discomfort (57). This has led to the assessment of smaller-bone IPC (10-14F) for drainage and administration of sclerosing agents (58-60).
Pleuroperitoneal shunting
For pts with trapped lung or for pts who failed to control an MPE with other interventions, pleuroperitoneal shunting remains an alternative approach (61).
Pleurectomy
There is not sufficient evidence to recommend this as an alternative to pleurodesis or IPC in recurrent effusions or trapped lung.
Suicide gene therapy
Suicide gene therapy is the methodology where viral or bacterial genes are inserted into tumors cells and consequently modify a non-cytotoxic drug into a cytotoxic. The non-cytotoxic agent has already been previously administered either intravenously or locally. The two major systems that have been extensively been used are the herpes simplex virus thymidine kinase gene (HSV-tk) and cytosine deaminase (CD) of Escherichia coli. The first converts the pro-drug ganciclovir (GCV) to GCV monophosphate and thereafter to GCV trisphosphate inside the cells and the second 5-Fluorocytosine (5-FC) to the cytotoxic agent 5-Fluoracil (5-FU). The method of CD has been previously used with positive results in patients with MPE from NSCLC and SCLC. An algorithm regarding the methodology of administration of this therapy along with the criteria of the possible candidates has been previously proposed (62). However, still larger studies still remain to elicit whether this method will be used as a local treatment of lung cancer or it will be used as another method of pleurodesis. Certainly the cost-effectiveness of these two approaches has to be considered along with the effectiveness.
Conclusions
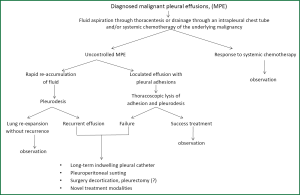
MPE is a common manifestation in patient with advanced lung cancer and other cancers. Therapy primary is directed to control symptoms and improve the quality of life rather than to cure the disease. Careful evaluation of the effusion to establish its etiology and patient treatment customization is required in order to decrease the volume of intrapleural fluid, to control the associated symptoms and to improve the quality of life and the survival. Talc pleurodesis (sterile asbestos-free graded, particle size >15 μm) still remains the treatment of choice in patients with MPE resistant to chemotherapy (Figure 6).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chernow B, Sahn SA. Carcinonomatous involvement of the pleura: an analysis of 96 patients. Am J Med 1977;63:695-702. [PubMed]
- Sahn SA. Malignant pleural effusions. Semin Respir Crit Care Med 2001;22:607-16. [PubMed]
- Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [PubMed]
- Noppen M. Normal volume and cellular contents of pleural fluid. Curr Opin Pulm Med 2001;7:180-2. [PubMed]
- Fenton KN, Richardson JD. Diagnosis and management of malignant pleural effusions. Am J Surg 1995;170:69-74. [PubMed]
- Antunes G, Neville E. Management of malignant pleural effusions. Thorax 2000;55:981-3. [PubMed]
- Johnston WW. The malignant pleural effusion: a review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [PubMed]
- Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Vancer Staging Project: proposals for revision of the M descriptors in the forth-coming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol 2007;2:686-93. [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [PubMed]
- Assi Z, Caruso JL, Herndon J, et al. Cytologically proved malignant pleural effusions: distribution of transudates and exudates. Chest 1998;113:1302-4. [PubMed]
- Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985;60:158-64. [PubMed]
- Woenckhaus M, Grepmeier U, Werner B, et al. Microsatellite analysis of pleural supernatants could increase sensitivity of pleural fluid cytology. J Mol Diagn 2005;7:517-24. [PubMed]
- Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol 1991;4:320-4. [PubMed]
- Ong KC, Indumathi V, Poh WT, et al. The diagnostic yield of pleural fluid cytology in malignant pleural effusions. Singapore Med J 2000;41:19-23. [PubMed]
- Sallach SM, Sallach JA, Vasquez E, et al. Volume of pleural fluid required for diagnosis of pleural malignancy. Chest 2002;122:1913-7. [PubMed]
- Benlloch S, Galbis-Caravajal JM, Martin C, et al. Potential diagnostic value of methylation profile in pleural fluid and serum from cancer patients with pleural effusion. Cancer 2006;107:1859-65. [PubMed]
- Edmondstone WM. Investigation of pleural effusion: comparison between fibreoptic thoracoscopy, needle biopsy and cytology. Respir Med 1990;84:23-6. [PubMed]
- Lee YC, Light RW. Management of malignant pleural effusions. Respirology 2004;9:148-56. [PubMed]
- Maskell NA, Butland RJ. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003;58:ii8-17. [PubMed]
- Hung MS, Lin CK, Leu SW, et al. Epidermal growth factor receptor mutations in cells from non-small cell lung cancer malignant pleural effusions. Chang Gung Med J 2006;29:373-9. [PubMed]
- Brock MV, Hooker CM, Yung R, et al. Can we improve the cytologic examinations of malignant pleural effusions using molecular analysis? Ann Thorac Surg 2005;80:1241-7. [PubMed]
- Debeljak A, Kecelj P, Triller N, et al. Talc pleurodesis: comparison of talc slurry instillation with thoracoscopic talc insufflations for malignant pleural effusions. J BUON 2006;11:463-7. [PubMed]
- Margaritora S, Cesario A, Vita ML, et al. Single versus, multiple access video-assisted thoracic surgery in the treatment of malignant pleural effusion. Eur J Cardiothorac Surg 2007;32:397-author reply 397-8. [PubMed]
- Stefani A, Natali P, Casali C, et al. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg 2006;30:827-32. [PubMed]
- Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management of malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg 2006;29:829-38. [PubMed]
- Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58:ii29-38. [PubMed]
- Emad A, Rezaian GR. Treatment of malignant pleural effusions with a combination of bleomycin and tetracycline. A comparison of bleomycin or tetracycline alone versus a combination of bleomycin and tetracycline. Cancer 1996;78:2498-501. [PubMed]
- Freitas S, Jones J, Cordeiro C, et al. Pleurodesis with tetracycline or with talc-Variables in terms of effectiveness. Rev Port Pneumol 2005;11:13-4.
- Martínez-Moragón E, Aparicio J, Rogado MC, et al. Pleurodesis in malignant pleural effusions: a randomized study of tetracycline versus bleomycin. Eur Respir J 1997;10:2380-3. [PubMed]
- Villanueva AG, Gray AW Jr, Shahian DM, et al. Efficacy of short-term versus long-term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994;49:23-5. [PubMed]
- Haddad FJ, Younes RN, Gross JL, et al. Pleurodesis in patients with malignant pleural effusions: talc slurry or bleomycin? Results of a prospective randomized trial. World J Surg 2004;28:749-53; discussion 753-4. [PubMed]
- Kilic D, Akay H, Kavukcu S, et al. Management of recurrent malignant pleural effusion with chemical pleurodesis. Surg Today 2005;35:634-8. [PubMed]
- Ong KC, Indumathi V, Raghuram J, et al. A comparative study of pleurodesis using talc slurry and bleomycin in the management of malignant pleural effusions. Respirology 2000;5:99-103. [PubMed]
- Patz EF Jr, McAdams HP, Erasmus JJ, et al. Sclerotherapy for malignant pleural effusions: a prospective randomized trial of bleomycin vs doxycycline with small-bore catheter drainage. Chest 1998;113:1305-11. [PubMed]
- Sartori S, Tassinari D, Ceccotti P, et al. Prospective randomized trial of intrapleural bleomycin versus interferon alfa-2b via ultrasound-guided small-bore chest tube in the palliative treatment of malignant pleural effusions. J Clin Oncol 2004;22:1228-33. [PubMed]
- Barbetakis N, Antoniadis T, Tsilikas C. Results of chemical pleurodesis with mitoxantrone in malignant pleural effusion from breast cancer. World J Surg Oncol 2004;2:16. [PubMed]
- Barbetakis N, Vassiliadis M, Kaplanis K, et al. Mitoxantrone pleurodesis to palliate malignant pleural effusion secondary to ovarian cancer. BMC Palliat Care 2004;3:4. [PubMed]
- Schmidt M, Schaarschmidt G, Chemaissani A. Pleurodesis in malignant pleural effusion: bleomycin vs mitoxantrone. Pneumologie 1997;51:367-72. [PubMed]
- Heffner JE, Klein JS. Recent advances in the diagnosis and treatment of malignant pleural effusions. Mayo Clin Proc 2008;83:235-50. [PubMed]
- Dasmahapatra KS, Citrin P, Hill GJ, et al. A prospective evaluation of 5-fluoroucasil plus cisplatin in advanced squamous-cell cancer of the head and neck. J Clin Oncol 1985;3:1486-9. [PubMed]
- Seto T, Ushijima S, Yamamoto H, et al. Intrapleural hypotonic cisplatin treatment for malignant pleural effusion in 80 patients with non-small-cell lung cancer: a multi-institutional phase II trial. Br J Cancer 2006;95:717-21. [PubMed]
- Shoji T, Tanaka F, Yanagihara K, et al. Phase II study of repeated intrapleural chemotherapy using implantable access system for management of malignant pleural effusion. Chest 2002;121:821-4. [PubMed]
- Hoffer FA, Hancock ML, Hinds PS, et al. Pleurodesis for effusions in pediatric oncology patients at end of life. Pediatr Radiol 2007;37:269-73. [PubMed]
- Kuzdzał J, Sladek K, Wasowski D, et al. Talc powder vs doxycycline in the control of malignant pleural effusion: a prospective randomized trial. Med Sci Monit 2003;9:P154-9. [PubMed]
- Porcel JM, Salud A, Nabal A, et al. Rapid pleurodesis with doxycycline through a amll-bore catheter for the treatment of metastatic malignant pleural effusions. Support Care Cancer 2006;14:475-8. [PubMed]
- Ohta Y, Shimizu Y, Matsumoto I, et al. Management of malignant pleural effusion by multimodality treatment including the use of paclitaxel administered by 24-hour intrathoracic infusion for patients with carcinomatous pleuritis. J Exp Clin Cancer Res 2006;25:15-9. [PubMed]
- Perng RP, Chen YM, Wu MF, et al. Phase II trial of intrapleural paclitaxel injection for non-small-cell lung cancer patients with malignant pleural effusions. Respir Med 1998;92:473-9. [PubMed]
- Balassoulis G, Sichletidis L, Spyratos D, et al. Efficacy and safety of erythromycin as sclerosing agent in patients with recurrent malignant pleural effusion. Am J Clin Oncol 2008;31:384-9. [PubMed]
- Miller Q, Meschter C, Neumaster T, et al. Comparison of pleurodesis by erythromycin, talc, doxycycline and diazepam in a rapid model. J Surg Educ 2007;64:41-5. [PubMed]
- Tang X, Cheng D. The value of erythromycin pleurodesis in the treatment of malignant pleural effusions. Hua Xi Yi Ke Da Xue Xue Bao 1997;28:437-9. [PubMed]
- Foresti V. Intrapleural Corynebacterium parvum for recurrent malignant pleural effusions. Respiration 1995;62:21-6. [PubMed]
- Olivares-Torres CA, Laniado-Laborin R, Chavez-Garcia C, et al. Iodopovidone pleurodesis for recurrent pleural effusions. Chest 2002;122:581-3. [PubMed]
- Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 2007;369:1535-9. [PubMed]
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402-19. [PubMed]
- Antony VB, Rothfuss KJ, Godbey SW, et al. Mechanism of tetracycline-hydrochloride-induced pleurodesis. Am Rev Respir Dis 1992;146:1009-13. [PubMed]
- Belani C, Ziskind A, Dhawan M, et al. Management of malignant pleural and pericardial effusions. Comprehensive Textbook of Thoracic Oncology, Williams and Wilkins 1996;43:880-905.
- Owen S, Gould D. Underwater seal chest drains: the patient’s experience. J Clin Nurs 1997;6:215-25. [PubMed]
- Clementsen P, Evald T, Grode G, et al. Treatment of malignant pleural effusion: pleurodesis using a small percutaneous catheter. A prospective randomized study. Respir Med 1998;92:593-6. [PubMed]
- Goff BA, Mueller PR, Muntz HG, et al. Small chest-tube drainage followed by bleomycin sclerosis for malignant pleural effusions. Obstet Gynecol 1993;81:993-6. [PubMed]
- Chen YM, Shih JF, Yang KY, et al. Usefulness of pig-tail catheter for palliative drainage of malignant pleural effusions in cancer patients. Support Care Cancer 2000;8:423-6. [PubMed]
- Genc O, Petrou M, Ladas G, et al. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. Eur J Cardiothorac Surg 2000;18:143-6. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]