Early oral nutrition plays an active role in enhanced recovery after minimally invasive esophagectomy
The incidence of esophageal cancer has been increasing over the past two decades (1). Despite improvement in treatment options, such as chemotherapy and radiotherapy, for patients with localized thoracic esophageal cancer, esophagectomy with regional lymph node dissection remains the mainstay of curative modality. Morbidity is a major concern during the follow-up period because of the invasive nature of esophagectomy and the complex operative procedures involved. However, recent studies have demonstrated a volume-outcome relationship for esophageal surgery; morbidity and mortality significantly decrease in high-volume hospitals (2). Improved outcomes in high-volume hospitals partly depend on thorough perioperative management by a multidisciplinary team using agreed written protocols throughout the patient’s hospital stay. In the late 1990s, Kehlet et al. advocated a fast-track multimodal program in colon cancer surgery and demonstrated both decreased postoperative complications and shortened length of hospital stay (3). This concept, originally developed to allow for a stress-free operation with minimal pain, has been shown to improve surgical outcomes based on understanding of the physiological and psychological role of various components of the surgical stress response that can be modified during perioperative period and has been applied to other cancers as enhanced recovery after surgery (ERAS). Cerfolio et al. first introduced this concept to esophageal surgical practice (4). Recent studies have demonstrated the feasibility and benefits of the ERAS protocol in esophageal cancer surgery (5), and now the ERAS protocol is used not only to enhance patient recovery but also to reduce hospital costs.
In the study, Sun and coworkers conducted a single-center, open-labeled, randomized control trial to evaluate the impact of early oral feeding on postoperative course after minimally invasive esophagectomy (MIE) (6). Consistent with the above descriptions, the aim of this study was to determine the utility of MIE in the ERAS protocol. This study included 280 patients who underwent MIE, with patients divided into two groups: patients who were allowed to eat a regular diet on POD 1 (n=140; early oral feeding; EOF group) and patients who were restricted to eat until POD 6 and fed via a nasogastric or nasoenteral tube (n=140; late oral feeding; LOF group). Cardiac, respiratory, and gastrointestinal complications after MIE were assessed in both groups as the primary endpoint, with non-inferiority observed for the EOF group compared with the LOF group in terms of postoperative complications. The incidence of anastomotic leakage was similar between the two groups (EOF group, 3.6%; LOF group, 4.3%). Consequently, the EOF group had a significantly shorter length of hospital stay. The authors also revealed both early recovery of bowel movement and higher quality of life (QOL) status in the EOF group.
Early enteral nutrition after surgery is known to be a key component of the ERAS protocol, and previous studies have shown that early enteral feeding postoperatively preserves gut mucosal integrity and improves immunological functions (7). Considering the high risk of anastomotic leakage after esophagectomy, enteral nutrition is predominantly administered directly into the jejunum by a surgically placed jejunostomy or nasojejunal tube, not via oral intake. On the other hand, early oral intake has been shown to be feasible and safe in other gastrointestinal cancers (8). Furthermore, both artificial feeding routes, jejunostomy and nasojejunal tubes, are associated with additional costs and complications. Accordingly, the ERAS protocol recommends that the unnecessary placement of drains and feeding tubes should be avoided. Lassen et al. also conducted a randomized controlled trial comparing patients who were allowed to eat a normal diet at will with patients managed with a routine of nil by mouth and enteral tube nutrition after upper GI surgery, including esophagectomy. The results of this study demonstrated that allowing patients to eat a normal diet at will from the first day did not increase morbidities, including anastomotic leakage (9). However, only eight patients with esophagectomy were included in this study. Therefore, the study by Sun et al. is the first to describe the early oral intake after esophagectomy using large sample size, and suggests a clinical need to reassess the feasibility and safety of early oral intake after esophagectomy.
Although the authors selected the patients without comorbidities and organ dysfunction preoperatively and did not comment on their own multidisciplinary ERAS team in the study, patients who undergo esophageal surgery should be managed during pre-, intra-, and postoperative periods under the ERAS protocol. Comorbidities and organ dysfunction are occasionally associated with serious postoperative complications. Preoperative optimization of organ function can reclassify a patient from a high-risk group to a relatively low-risk group. Furthermore, patients and relatives are informed of the rehabilitation program after esophageal surgery and expected outcomes by a multidisciplinary team during the preoperative visit. In particular, education regarding a respiratory rehabilitation and swallowing training is important for patients who have undergone esophageal cancer surgery. We previously demonstrated that a preoperative care bundle could successfully prevent postoperative pneumonia after esophagectomy (10). Preservation of gastrointestinal function is also a key component of the ERAS protocol. Mechanical bowel preservation is selectively used to facilitate bowel handling, especially when reconstruction using the colon is planned after esophagectomy. Clear liquid intake should not be routinely prohibited until several hours preoperatively, and early enteral nutrition should be enforced. As Sun and coworkers demonstrated, early postoperative feeding can improve the recovery of peristalsis, protects gut mucosal barrier function, and strengthens the immune response. However, because anastomosis is performed at the neck or upper mediastinum between the esophagus and gastric tube after esophagectomy, most surgeons prefer enteral tube feeding distal to the anastomosis for nutritional support. Even after esophagectomy, surgeons concerned with maintaining or increasing physiological and psychological patient activity, and unnecessary use of drains and nasogastric tubes should be avoided.
According to the ERAS protocol, patients are encouraged to ambulate immediately postoperatively. The ERAS protocol recommends that surgeons do not perform unnecessarily long skin incisions in order to reduce postoperative pain. Long thoracic and abdominal incisions occasionally cause intolerable postoperative pain that interferes with early mobilization. Accordingly, active pain control is critical for enhancing recovery after esophagectomy. Previous reports have demonstrated that thoracic epidural analgesia reduces postoperative pain and improves patient outcomes after esophagectomy (11). Epidural analgesia can support early postoperative mobilization, effective coughing, and vigorous physiotherapy. Furthermore, thoracic epidural anesthesia improves microcirculation of the gastric tube after esophagectomy and may decrease the incidence of anastomotic leakage (12). Although epidural analgesia is most effective for the control of the postoperative pain, skin incision length is known to be associated with postoperative pain, and epidural anesthesia is unable to completely control severe pain after esophagectomy.
As the authors performed, MIE has the potential to allow a quicker return to normal function and decrease morbidity among patients after esophagectomy (13) (Figure 1). Since Cuschieri et al. first reported the use of thoracoscopic esophagectomy for the treatment of esophageal cancer in 1992 (14), many surgeons have been interested in performing the procedure. In conjunction with the wide acceptance of the ERAS protocol, the number of MIE procedures that are being performed has been increasing, and large single-center studies have demonstrated that MIE may have some functional advantages, especially regarding respiratory function (15). Meta-analyses using individual institute reports comparing MIE with transthoracic esophagectomy have shown that MIE is associated with decreased operative blood loss, shorter length of intensive care unit and hospital stays, and reduced incidence of postoperative respiratory complications (16,17). On the other hand, results from several nationwide database analyses have been disappointing, with results demonstrating no reduction in postoperative respiratory complications and higher reoperation or reintervention rates with MIE (18,19). However, these unexpected results may be attributable to the inclusion of a wide range of patients, surgeons, and hospitals in the nationwide database analyses. Therefore, we have recognized the necessity of a prospective study that will demonstrate lower invasiveness and improved QOL associated with MIE compared with transthoracic esophagectomy. However, multicenter randomized trials were not reported until quite recently because of the diversity of operative techniques used for MIE and the surgeons’ experience levels. After standardization of surgical techniques and perioperative management using the ERAS protocol, Biere et al. reported the results of a multicenter randomized control trial that compared MIE with the patient in the prone position and transthoracic esophagectomy (20). The results of this study demonstrated the apparent short-term benefits of MIE, such as fewer respiratory complications and shorter length of hospital stay, and also demonstrated that the reoperation rate was similar in both groups. Patients in the MIE group were satisfied with their QOL, with better physical status, better ability to speak, and lesser pain. Luketich et al. conducted a prospective phase II multicenter trial demonstrating the short-term feasibility and safety of MIE (21). Although the usefulness of laparoscopic gastric mobilization combined with thoracoscopic surgery and differences between the left decubitus position and prone position are issues that require further assessment, MIE is now considered to be one of the key ERAS factors that can help reduce postoperative pain and enhance postoperative recovery after esophagectomy.
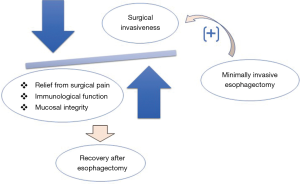
Sun et al. demonstrated the contribution of EOL and MIE to enhance postoperative management. To our knowledge, apparent benefits of MIE to the ERAS protocol have not yet been demonstrated. Recent studies have reported relatively shorter length of hospital stay in patients who underwent MIE compared with those who underwent transthoracic esophagectomy; however, the lengths of hospital stay for these patients were still longer than those for patients who underwent other gastrointestinal cancer surgeries. The length of hospital stay predominantly depends on the setting of the date to start first diet after gastrointestinal surgery. If early oral intake after esophagectomy is possible similar to other gastrointestinal surgeries, the length of hospital stay can be shortened without further interventions. Theoretically, MIE has the potential benefit of allowing the introduction of early oral intake because of significant reductions of surgical invasiveness. However, contrary to recent ERAS strategies being used in other gastrointestinal cancer surgeries, many esophageal surgeons remain reluctant to introduce early oral intake even after MIE. The reasons for continuing traditional nil by mouth after esophagectomy are concerns regarding anastomotic leakage and aspiration pneumonia associated with recurrent laryngeal nerve paralysis (22). In the study, Sun et al. demonstrated that anastomotic adverse events and pneumonia were not increased in the EOF group. In addition, the authors reported a rat model in which early postoperative oral intake accelerates esophagogastric anastomotic healing (23). Therefore, the routine nil by mouth protocol after esophagectomy may not be justified, and early oral intake may have utility in improving postoperative recovery following MIE.
Although incidence of anastomotic leakage in the study was relatively lower, the anastomosis between the cervical esophagus and gastric conduit, commonly used for reconstruction after esophagectomy, is more likely to leak than other gastrointestinal anastomoses, and is consequently associated with higher postoperative mortality. Prevention of anastomotic leakage can improve the postoperative course of patients who undergo esophagectomy. Among several factors, such as preoperative nutritional status, reconstructed route, and site or technique of the anastomosis, there is a high probability that ischemia of the gastric conduit may contribute to anastomotic leakage. Perfusion and viability of the gastric conduit is commonly subjectively determined by clinical judgment according to color, movement, and pulsation of the vessels. If substantial intraoperative or postoperative measurement system of tissue blood flow is established, surgeons can obtain objective and reliable information to make decisions regarding the most appropriate management. We recently demonstrated near-infrared fluorescence using ICG as a promising intraoperative system for the assessment of gastric conduit wall blood flow, with an ability to predict anastomotic leakage after esophagectomy (24). Nishikawa et al. performed postoperative endoscopic examinations and demonstrated an association between ischemic change at the anastomosis and anastomotic complications (25). These objective parameters can be helpful for the safe management of early oral intake protocols after esophagectomy.
Sun and coworkers demonstrated higher QOL status in the EOF group. Early postoperative feeding may also be associated with higher QOL. In the study, all QOL assessment scores, such as global QOL, and physical, emotional, and social functions, were higher in the EOF group than in the LOF group. As MIE has the benefit of reducing postoperative pain, the observed differences between the EOF and LOF groups, particularly regarding pain, were difficult to explain. The exact mechanism underlying the association between EOF and QOL has yet to be elucidated. Early oral intake may have psychological advantages and promote a short-term postoperative course. However, the higher QOL scores in the EOF group disappeared at 8 weeks postoperatively.
The findings of the study by Sun et al. pose several issues that require further investigation. First, this study was conducted using selective patients and the early resumption of oral nutrition may be associated with multiple factors, such as MIE, no nasogastric drainage, no enteral feeding tube, and early mobilization. Accordingly, further studies including patients with comorbidities and open esophagectomy should be conducted to investigate the utility of the ERAS protocol in EOF. Second, definitive indications of EOF after esophagectomy should be established. As we demonstrated the usefulness of ICG fluorescence for assessing the blood flow of the gastric conduit, objective evaluation systems that can identify patients with a high risk of anastomotic leakage should be utilized after esophagectomy as the incidence of esophagogastric anastomotic leakage remains relatively higher compared with other gastrointestinal surgeries. Third, objective parameters that reflect the surgical invasiveness of MIE also require further investigation. MIE is considered to be a less invasive procedure that preserves the immunological condition; however, previous studies did not use surrogate or predictive parameters. As with oncological evaluation during chemotherapy or chemoradiotherapy for malignant tumors, representative evaluation systems or surrogate markers are required to assess the efficacy of MIE.
In conclusion, MIE was considered to be less invasive and contribute to the recovery of patients following esophagectomy until recently because of its use of small skin incisions. However, there is a lack of scientific evidence demonstrating an association between MIE and the ERAS protocol. Accordingly, there is a clinical need for studies evaluating the efficacy of MIE in improving the postoperative course of patients with esophageal cancers and developing surrogate markers that indicate the lower invasiveness of MIE.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Bass CS, et al. Fast tracking after Ivor Lewis esophagogastrectomy. Chest 2004;126:1187-94. [Crossref] [PubMed]
- Pisarska M, Małczak P, Major P, et al. Enhanced recovery after surgery protocol in oesophageal cancer surgery: Systematic review and meta-analysis. PLoS One 2017;12:e0174382. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy: An Open-label, Randomized, Controlled, Noninferiority Trial. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Chen L, Sun L, Lang Y, et al. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016;16:449. [Crossref] [PubMed]
- Ni TG, Yang HT, Zhang H, et al. Enhanced recovery after surgery programs in patients undergoing hepatectomy: A meta-analysis. World J Gastroenterol 2015;21:9209-16. [Crossref] [PubMed]
- Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008;247:721-9. [Crossref] [PubMed]
- Hiramatsu T, Sugiyama M, Kuwabara S, et al. Effectiveness of an outpatient preoperative care bundle in preventing postoperative pneumonia among esophageal cancer patients. Am J Infect Control 2014;42:385-8. [Crossref] [PubMed]
- Watson A, Allen PR. Influence of thoracic epidural analgesia on outcome after resection for esophageal cancer. Surgery 1994;115:429-32. [PubMed]
- Lázár G, Kaszaki J, Abrahám S, et al. Thoracic epidural anesthesia improves the gastric microcirculation during experimental gastric tube formation. Surgery 2003;134:799-805. [Crossref] [PubMed]
- Koyanagi K, Ozawa S, Tachimori Y. Minimally invasive esophagectomy performed with the patient in a prone position: a systematic review. Surg Today 2016;46:275-84. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. [Crossref] [PubMed]
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Mamidanna R, Bottle A, Aylin P, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012;255:197-203. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Koyanagi K, Igaki H, Iwabu J, et al. Recurrent Laryngeal Nerve Paralysis after Esophagectomy: Respiratory Complications and Role of Nerve Reconstruction. Tohoku J Exp Med 2015;237:1-8. [Crossref] [PubMed]
- Tadano S, Terashima H, Fukuzawa J, et al. Early postoperative oral intake accelerates upper gastrointestinal anastomotic healing in the rat model. J Surg Res 2011;169:202-8. [Crossref] [PubMed]
- Koyanagi K, Ozawa S, Oguma J, et al. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore) 2016;95:e4386.
- Nishikawa K, Fujita T, Yuda M, et al. Early postoperative endoscopy for targeted management of patients at risks of anastomotic complications after esophagectomy. Surgery 2016;160:1294-301. [Crossref] [PubMed]


