Endoscopic naso-leakage drainage: a safe and effective method for the management of intrathoracic anastomotic leakage after esophagectomy
Introduction
Postoperative anastomotic leakage, which includes cervical leakage and intrathoracic leakage, is one of the most common complications after esophagectomy and occurs in 5% to 30% of cases (1-3). Thoracic anastomotic leakage shows a wide variety of clinical presentations, ranging from clinically silence to severe sepsis (4,5). As for cervical leakage, the incidence rate is 10% to 20%, but leakage-related death barely occurs. Nevertheless, intrathoracic leakage carries mortality rates as high as 30%, with the incidence of 5% to 10% (6). Thus, an efficacious management of intrathoracic leakage becomes extremely urgent (7,8).
For those patients who suffering intrathoracic leakage, existing intervention varies from surgical options to non-surgical options. Traditional surgical interventions have been proved invasive and have been increasingly replaced by non-surgical ways (7,8). Meanwhile, as for non-surgical options, chest drainage is the most essential method, but usually cannot get sufficient drainage, especially in the mediastinum and top of the chest. Endoscopic interventions such as endoscopic stenting (9,10), endoluminal vacuum therapy (EVT) (11) and endoscopic clamping therapy do show certain effectiveness, but it is also associated with severe complications with a high mortality which could not be ignored. Endoscopic stenting, for example, might cause aortic erosion, immigration of stent and enlargement of leakage (9,10). Moreover, treatment of antibiotics and enteral nutrition are well used today but they are not crucial, and could seldom come into effect alone. Hence, efficacious options for intrathoracic leakage need urgently to be developed to achieve a better outcome.
On managing the intrathoracic leakage, we came to know that the crucial point is how to get an adequate drainage, for insufficient drainage might lead to fatal outcomes, such as systemic inflammatory response syndrome (SIRS), sepsis, multiple organ failure, vascular erosion and esophago-bronchial leakage (1-5). In addition, an appropriate rinse may helpful for the closure of leakage. Therefore, we performed endoscopic naso-leakage drainage (ENLD) in management of intrathoracic anastomotic leakage (IAL), not only to drain the vomica from inside, especially where the chest tube could not easily reach, but also to rinse the vomica. This study retrospectively analyzed the safety and effect of ENLD for the patients with IAL.
Methods
Patients
We conducted a retrospective research based on all 67 patients who suffered IAL and received a non-surgical intervention after esophagectomy with only intrathoracic anastomosis in Zhongshan Hospital, Shanghai, during the period of Jun 2011 to Nov 2016. All these patients were diagnosed IAL by CT scan together with endoscopy and radiography alternatively.
These 67 patients received a routine conservative therapy, including apply of antibiotics and enteral nutrition. Among these 67 patients, moreover, 38 patients were treated by chest drainage (CD group, n=38) alone, while 29 were treated by ENLD (ENLD group, n=29) with or without chest drainage (Figure 1).
Diagnosis of IAL
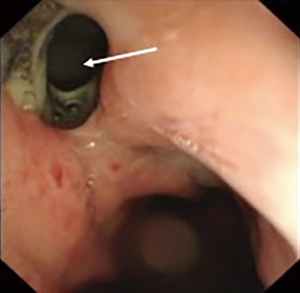
Patients are highly suspected of IAL with the following indications: persistent fever over 38.5°C, increased white blood cell count and turbid purulent drainage fluid. After that, a chest contrast CT scan (Figure 2) with iodine swallow is carried out first to evaluate the details of vomica in both pleural cavity and mediastinum, while a further definite diagnosis requires the fiber gastroscope examination (Figure 3). On being diagnosed with IAL with an abscess that could not be reached by chest tube in pleural cavity and mediastinum, ENLD is considered at the same time of fiber gastroscope examination.
Performance of the procedure
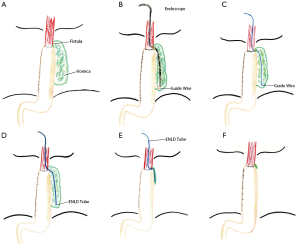
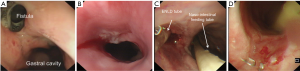
At the very beginning of ENLD, we evaluated the accurate situation of leakage by CT scan, after the confirmation of the leakage (Figure 4A). Then an ultra-slim endoscope was inserted through nasal cavity, went through the leakage and finally down to the bottom of vomica. Under endoscope, rinse and suction were performed repeatedly to get an elimination of pus liquid and necrosis tissues. After that, we inserted a guide wire with quite a soft tip down to the bottom of vomica (Figure 4B), and withdraw the endoscope with a caution not to pull out the wire (Figure 4C). Finally, the drain tube was placed via the guide wire, and after that the guide wire was removed (Figure 4D). The drain tube was connected to a vacuum device and we rinsed the vomica twice a day, as well as to keep the tube unobstructed. When CT scan showed that the vomica diminished and the drainage became clean, we began to pull out the tube gradually about 2–3 centimeters per day (Figure 4E). Eventually, we used endoscopy to confirm the healing of the leakage, after the complete removal of the drain tube (Figure 4F). Two cases were taken as an example of the entire healing course after ENLD (Figures 5-7).
Case 1 (Figure 5)
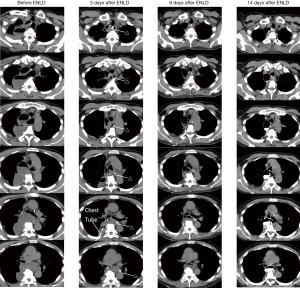
A male patient, 8 days after Ivor-Lewis esophagectomy, had a high fever of 39 °C. CT showed abscess in the mediastinum and right chest. Three days after ENLD, we can see the tube was in the mediastinum, we also placed a right chest tube. These two abscesses were already smaller than before. Nine days later, the mediastinal abscess almost disappeared, just leaving the ENLD tube. Also, the right chest abscess became smaller. Sixteen days later, the tube had been pulled out.
Case 2 (Figure 6)
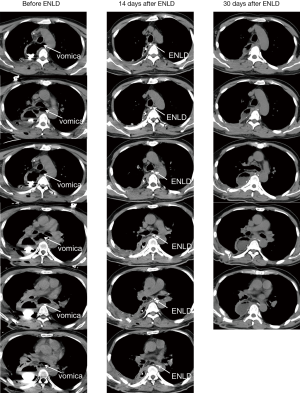
A male patient, 6 days after Ivor-Lewis, also had a high fever. CT showed there was a vomica in the mediastinum. We also placed a ENLD tube to drain and flush. Fourteen days later, the vomica almost disappeared, just leaving the tube. Thirty days after ENLD, the tube had already been pulled out and the mediastinum was clear.
Figure 7A shows the anastomotic leakage before ENLD (case 1). Twenty-four days after ENLD, the healing of the leak was confirmed by endoscopy, just leaving a scar (case 1, Figure 7B). Figure 7C shows how ENLD tube was placed, the left tube was the ENLD tube into the leakage, the right one was the feeding tube in the gastral cavity to small intestine (case 2). Figure 7D shows the healing of the leak 35 days later, also leaving a big scar there (case 2).
Outcome parameters
The following outcome parameters were evaluated and compared in these 67 patients in CD group together with ENLD group: incidence of procedure-related complications, SIRS and vascular erosion, time for healing of the leakage, duration of fever, duration of antibiotics usage and mortality.
Statistical analysis
The demographics and clinical characters of these patients between CD group and ENLD group were analyzed, while the following outcome parameters were evaluated and compared between two groups: procedure-related complications, SIRS, vascular erosion, in-hospital mortality, healing course (days), duration of fever (days), duration of antibiotics usage (days). Chi-square testing was used for categorical parameters (or Fisher’s exact testing in case of small count), and Mann-Whitney U testing was used for continuous parameters. Statistical analysis was performed using IBM SPSS Statistics version 24.0 (IBM Corp., New York, USA). A P value of <0.05 was considered statistically significant.
Results
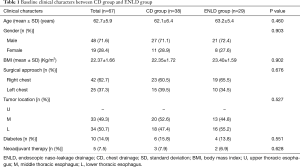
Between Jun 2011 and Nov 2016, a total of 1,527 patients underwent curative esophagectomy with intrathoracic anastomosis in Zhongshan Hospital, Fudan University, of whom 67 (4.4%) patients suffered IAL after surgery and received non-surgical intervention. The baseline demographics and clinical characters were shown in the Table 1. Among these 67 patients, 38 of 67 patients received chest drain (CD group, n=38, 56.7%), and 29 received ENLD (ENLD group, n=29, 43.3%) with or without chest drainage. No significant difference of in patient background existed between CD group and ENLD group, including age, gender, body mass index (BMI), surgical approach, tumor location, diabetes and neoadjuvant therapy (Table 1).
Full table
After diagnosed with IAL, all these 67 patients received standard antibiotics therapy and eternal nutrition immediately. Despite no procedure-related complications were found in both group, the CD group had more severe complications and more negative clinical outcomes than the ENLD group. In the CD group, 35 (92.1%) patients were complete cured finally but severe complications due to insufficient drainage occurred in 7 patients: 5 (13.2%) patients suffered SIRS and one of them died in the last, while the other 2 (5.3%) patients suffered vascular erosion and all died. The mortality of the CD group was 3 (7.9%). In the EBLD group, all 29 patients were cured finally and only 1 patient developed to SIRS without mortality, but the difference was not significant between the two groups because of small sample size. Notably, however, the healing course, duration of fever and antibiotics use have been observed remarkably shortened in the ENLD group in comparison with those in the CD group, with 44.2±18.3 vs. 60.5±27.7 days in healing course (P=0.008), 4.3±2.2 vs. 9.5±8.6 days in duration of fever (P=0.002) and 11.8±3.8 vs. 16.4±7.8 days in duration of antibiotics usage (P<0.001), which was statistically significant (Table 2).

Full table
Discussion
Fatal outcomes of IALs and non-surgical approaches
Anastomotic leakage remains a common and serious complication of esophagectomy, while intrathoracic leakage is the most dangerous type, certain fatal complications of which would sometimes be fatal, such as SIRS, sepsis, multiple organ failure, vascular erosion and esophago-bronchial leakage (1-5).
Over the past decades, with certain potent antibiotics coming into use and the progress made in field of nutrition support, the management of IAL becomes more efficacious and the mortality has reduced. However, these conservative interventions seem to be incapable to handle all the severe cases, while surgical intervention has been proved invasive and defective in consideration of the condition of patients suffering IAL (7,8). Hence, several non-surgical options have come insight these years, including endoscopic stenting, vacuum-assisted closure therapy (VACT) and naso-leakage drainage under X-ray fluoroscopy. Whereas, these non-surgical interventions sometimes show certain defects which might lead to a failing management and cause fatal outcomes (9-12). Therefore, a more effective and less invasive option with quite a safety to heal the leakage and reduce the mortality is urgently needed.
The key to manage IAL—adequate drainage and appropriate rinse
There are some places hard to be reached by chest tube, the most common of which is abscess in the mediastinum and the top of the chest. Hence, adequate drainage is essential in management of intrathoracic leakage to keep those patients from life-threatening complications and spare time to heal the leakage (1-5). In this study, patients with cervical anastomosis were excluded. As for a mediastinal vomica, we could easily open the cervical wound and insert a tube down into the mediastinum, which could have a similar effectiveness with ENLD.
Meanwhile, during the procedures of placing ENLD tube, immediate suction and irrigation of the abscess cavity under endoscopy was very helpful for removing pus and necrotic tissues, which could significantly reduce systemic inflammatory response. Furthermore, subsequent daily irrigation through ENLD tube could also shorten the course of healing of the IAL. In this study, the ENLD group had a significantly shorter healing course, duration of fever and antibiotics usage. According to Table 2, adding ENLD caused averagely a 29.4% shorter healing course in average, a 54.7% shorter duration of fever in average and a 22.0% shorter duration of antibiotics usage in average. More importantly, placing ENLD also showed no death or vascular erosion and less SIRS due to leakage than chest drainage alone, though the difference was not significant between the two groups because of small sample size. Thus, ENLD may be a more efficacious method than chest drainage alone in management of IALs.
Safety of ENLD: compared to endoscopic stenting
With a wide use in recent years, endoscopic stenting does show certain effectiveness, but it also comes to show the association with severe complications with a high mortality which could not be ignored. Schweigert et al. (13,14) reported 29 patients underwent endoscopic stenting after being diagnosed with IAL, and it turned out to have a pretty high stent-related complications rate. Of those 29 patients, 3 (10.3%) suffered aortic erosion, 1 (3.4%) suffered hemorrhage in the small intestine caused by migration, and 1 (3.4%) suffered obstruction of the airways. All these complications were deadly for a IAL patient. Moreover, stents could not fundamentally improve the insufficient drainage of the vomica, hence, the pus and necrotic tissues constantly inhibit healing of leakage. Oppositely, ENLD provides not only a constant drainage, but also a capacity to rinse to clear the vomica. In our study, no death due to IAL or ENLD-related complications were observed in ENLD group. Thus, on comparing to the endoscopic stenting, ENLD may be a safer endoscopic intervention and a more efficacious drainage and rinse option. Further studies containing larger sample size are still required.
Capacity to rinse and economical efficiency: compared to endoluminal vacuum therapy
EVT is based on the technique of VACT which has been used for over 20 years in treating contaminated soft tissue wounds, while Weidenhagen et al. (11) reported endoscopic vacuum-assisted closure of anastomotic leakages may help to overcome the limitations associated with intermittent endoscopic stenting and conventional drainage therapy. They used a specially designed synthetic sponge system which was put into the vomica under endoscope which could heal intrathoracic esophageal leakages in all six patients without any local complications. Moreover, Kuehn et al. proved EVT is a promising approach for postoperative, iatrogenic, or spontaneous lesions of the upper GI tract (15).
However, the crucial point of EVT is the application of a certain device, like sponge in the study of Weidenhagen et al. (11), which needs to be changed every 48 to 72 h through the mouth of the patient under endoscopy. This adds to patients’ discomfort by taking endoscopy every 2 to 3 days. In addition, the insertion of sponge system makes it unable to rinse easily without the help of endoscopy. Moreover, there is a hypothetical risk that the sponge system might erode larger mediastinal vessels, which would cause major hemorrhage. Meanwhile, all these devices and endoscopies every 2 to 3 days might cost much higher than ENLD in our study, which is not economically friendly.
It has been shown that leakage-associated mortality can be reduced by repeated endoscopic lavage and debridement together with adequate drainage (5). In comparison with EVT, ENLD provides both a convenient and a vital way to rinse the vomica as well as an adequate direct drainage without the help of endoscopy. At the same time, with less devices and endoscopies needed, ENLD shows a great economy during hospital stay.
Ease of operation: compared to naso-leakage drainage under X-ray fluoroscopy
Naso-leakage drainage under X-ray fluoroscopy has been used for over a decade, and developed to a superior option than other non-surgical interventions (16). Shuto et al. (12) reported a retrospective study with 50 patients suffering anastomotic leakage after esophagectomy, suggesting similar results to this study. They observed no reintervention or reoperation and all experienced complete cure in the naso-esophageal extraluminal drainage (NEED) group, with a 0% mortality. They came to the view that NEED under X-ray fluoroscopy and concomitant enteral nutritional support were less invasive and more effective methods to treat even major leakage after esophagectomy compared to chest drainage.
Whereas, ENLD is proved superior compared to NEED under X-ray fluoroscopy. In contrast with X-ray fluoroscopy which might not succeed sometimes, endoscopy provides a much better and direct view of the leakage and vomica which makes it more accurate, safer and easier to insert a tube. This leads to a pretty much higher achievement ratio, less procedure-related complications and radiation injury in both patients and physicians. Apart from that, ENLD permits rinse of the vomica under endoscopy immediately, which could control infection and improve the condition of patients at once. All these make ENLD a more promising option in management of IAL with a superiority to NEED under X-ray fluoroscopy .
Prospect and limitation
The ENLD provides an efficacious option in management of IALs by adequate drainage and appropriate rinse, considering its efficacy, safety, economy and ease of operation. To our experience, ENLD is capable of being used for intrathoracic leakage safely and effectively, especially for some hard-to-drain vomica, with no death observed during the period. In addition, with the proficiency of endoscopic intervention, the ENLD has a promising prospect to be widely adopted in the standard IAL management.
However, this study is a retrospective small-sample-sized research of single center, and the selection of patients was also not randomized. To define the clinical value of this new concept adequately, more experience and more prospective studies will be required that compare non-surgical interventions of IAL.
Conclusions
In conclusion, the study was a retrospective case-control study that demonstrated ENLD is a safe and effective non-surgical intervention in management of intrathoracic leakage, especially for some hard-to-drain vomica, considering its efficacy, safety, capacity to rinse, economy and ease of operation. Still, further studies are required to define its clinical value.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our ethics committee (NO. b2017-137). And written informed consent was obtained from all patients.
References
- Junemann-Ramirez M, Awan MY, Khan ZM, et al. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg 2005;27:3-7. [Crossref] [PubMed]
- Korst RJ, Port JL, Lee PC, et al. Intrathoracic manifestations of cervical anastomotic leaks after transthoracic esophagectomy for carcinoma. Ann Thorac Surg 2005;80:1185-90. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Pross M, Manger T, Reinheckel T, et al. Endoscopic treatment of clinically symptomatic leaks of thoracic esophageal anastomoses. Gastrointest Endosc 2000;51:73-6. [Crossref] [PubMed]
- Schubert D, Scheidbach H, Kuhn R, et al. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc 2005;61:891-6. [Crossref] [PubMed]
- van Rossum PS, Haverkamp L, Carvello M, et al. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017;30:1-8. [PubMed]
- Schaheen L, Blackmon SH, Nason KS., et al. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536-43. [Crossref] [PubMed]
- Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157-68. [Crossref] [PubMed]
- Tuebergen D, Rijcken E, Mennigen R, et al. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg 2008;12:1168-76. [Crossref] [PubMed]
- Kanatas AN, Aldouri A, Hayden JD. Anastomotic leak after oesophagectomy and stent implantation: a systematic review. Oncol Rev 2010;4:159-65. [Crossref]
- Weidenhagen R, Hartl WH, Gruetzner KU, et al. Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg 2010;90:1674-81. [Crossref] [PubMed]
- Shuto K, Kono T, Akutsu Y, et al. Naso-esophageal extraluminal drainage for postoperative anastomotic leak after thoracic esophagectomy for patients with esophageal cancer. Dis Esophagus 2017;30:1-9. [PubMed]
- Schweigert M, Solymosi N, Dubecz A, et al. Endoscopic stent insertion for anastomotic leakage following oesophagectomy. Ann R Coll Surg Engl 2013;95:43-7. [Crossref] [PubMed]
- Schweigert M, Solymosi N, Dubecz A, et al. One decade of experience with endoscopic stenting for intrathoracic anastomotic leakage after esophagectomy: brilliant breakthrough or flash in the pan? Am Surg 2014;80:736-45. [PubMed]
- Kuehn F, Schiffmann L, Janisch F, et al. Surgical Endoscopic Vacuum Therapy for Defects of the Upper Gastrointestinal Tract. J Gastrointest Surg 2016;20:237-43. [Crossref] [PubMed]
- Hu Z, Yin R, Fan X, et al. Treatment of intrathoracic anastomotic leak by nose fistula tube drainage after esophagectomy for cancer. Dis Esophagus 2011;24:100-7. [Crossref] [PubMed]