Worse survival after curative resection in patients with pathological stage I non-small cell lung cancer adjoining pulmonary cavity formation
Introduction
A possible association between lung cancer and bullous lung disease was first reported in 1951 by Bass and Singer (1). Pulmonary cavity formation such as bullae are an important risk factor for primary lung carcinoma (2-4). Although a few reports have addressed some peculiar and notable clinical features of lung cancer adjoining pulmonary bullae (5-7), this clinical association and its carcinogenic correlations are not well recognized. This study aimed to clarify the clinical characteristics and demonstrate the associated survival outcomes after curative resection in patients with early non-small cell lung cancer (NSCLC) adjoining pulmonary cavity formation.
Methods
Patient selection
We retrospectively reviewed 288 consecutive patients with pathological stage I NSCLC who underwent curative surgical resection including pneumonectomy, lobectomy and segmentectomy at our hospital from January 2010 to December 2014. We excluded 13 patients with the following characteristics: 3 patients who had received preoperative chemotherapy, radiotherapy, or both; 2 patients with uncurative resection, evidenced by macroscopically or microscopically observed residual cancer; and 4 patients with carcinoid, 3 patients with small cell lung cancer, and one patient with pleomorphic carcinoma (because they were diagnosed with different biological malignant features compared with NSCLC). Consequently, we enrolled the remaining 275 patients.
Definitions
These 275 patients with primary lung cancer were retrospectively examined by two thoracic surgeons through the re-evaluation of their chest computed tomography (CT) images. All the CT images had been previously assessed by a radiologist. By definition, a pulmonary cavity formation in the radiology literature is a gas-filled space, seen as a lucency, low-attenuation area, or air-containing space within the substance of the lungs; it results from the destruction, dilatation, and confluence of air spaces distal to the terminal bronchioles (8,9). Among the examined patients, we detected NSCLC adjoining pulmonary cavity formation in 12 (4.4%) patients. Two representative cases from our series are illustrated in Figures 1,2.
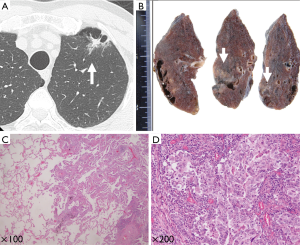
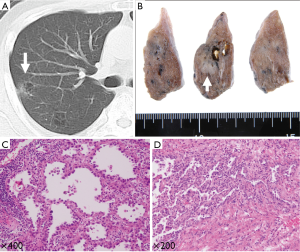
Data collection
Preoperative evaluation included physical examination, blood examination, chest radiography, and chest and abdomen CT. Brain CT or magnetic resonance imaging (MRI) and positron emission tomography (PET)-CT were performed if clinically indicated. Staging and pathological findings for lung cancer were determined according to the 7th TNM Classification for Lung and Pleural Tumors (10), the World Health Organization classification (11) and the IASLC/ATS/ERS classification system for lung adenocarcinoma (12).
All patients were followed up at our hospital every 3 months of the first year, every 6 months from the second to the 5th year, and annually thereafter on an outpatient basis; we aimed to continue follow-up for ≥5 years. The follow-up procedures included physical examination, chest radiography, and blood examination (including serum tumor markers). At follow-up, chest and upper abdomen CTs were routinely performed in every scheduled outpatient department. If any symptoms or signs of recurrence were detected, brain MRI and/or PET-CT were performed. Once a metastasis was discovered through physical examination and diagnostic imaging, the metastatic site was histologically or cytologically confirmed when clinically feasible. Metachronous second primary lung cancer was discriminated from a solitary pulmonary metastasis using the proposed criteria in the American College of Chest Physicians Lung Cancer Guidelines (13).
The hospital charts of all patients were reviewed to collect clinicopathological data including age, sex, smoking history, pack-years of smoking, spirometry, pathological T factor, histologic type, surgical procedures, relapse type, epidermal growth factor receptor (EGFR) status, overall survival (OS), and recurrence-free survival (RFS). OS was determined as the duration from the day of surgery until the day of death from all causes, with patients alive at the end of follow-up treated as censored cases. RFS was defined as the duration between the day of surgery and the day of recurrence of lung cancer or death from all causes. Patients who were alive and were without any evidence of recurrence at the end of the follow-up period were regarded as censored cases. This retrospective study was approved by the Institutional Review Board of the St Marianna University School of Medicine (No. 2233). The requirement for informed consent from the patients was waived because of the retrospective study design.
Statistical analysis
Summary statistics were constructed using frequencies and proportions for categorical data and means for continuous data. Patient characteristics were compared using χ2 test or Fisher’s exact test for categorical outcomes of continuous variables, as appropriate. OS and RFS were estimated using Kaplan-Meier method, and the survival differences between patient groups were determined using log-rank analysis. P values and hazard ratios in the multivariate analyses were calculated using Cox regression model. P values <0.05 were considered to be significant. All statistical calculations were performed using the SPSS statistical software package (version 21.0, SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
In total, 275 consecutively enrolled patients with pathological stage I NSCLC who underwent curative surgical resection at our hospital from January 2010 to December 2014 were assessed. Among them, 12 (4.4%) patients had primary lung cancer adjoining pulmonary cavity formation (group CF), and the remaining 263 patients with primary lung cancer were considered as controls (group C). Table 1 shows the various characteristics of the enrolled patients; no significant differences were observed between the two groups, except for the type of relapse. The rate of locoregional recurrence in group CF was tended higher than that in group CF (P=0.063). In the group CF, there are 8 adenocarcinomas including 1 lepidic predominant, 1 acinar predominant, 4 papillary predominant and 2 solid predominant, and 3 squamous cell carcinomas and 1 other.
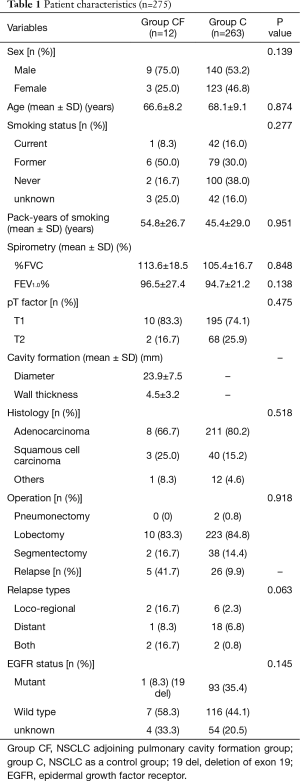
Full table
Survival
The median follow-up period for all 275 patients was 43.2 (range, 6.0–86.0) months. Of them, 6 (50.0%) patients in group CF and 19 (7.2%) patients in group C died during the study period. Besides, 6 (50.0%) patients in group CF and 32 (12.2%) patients in group C exhibited recurrence of the primary lung cancer. The cumulative OS in groups CF and C at 5 years was 37.0% and 91.7%, respectively (P<0.0001, Figure 3A); RFS in these groups at 5 years was 55.0% and 86.7%, respectively (P=0.002, Figure 3B).
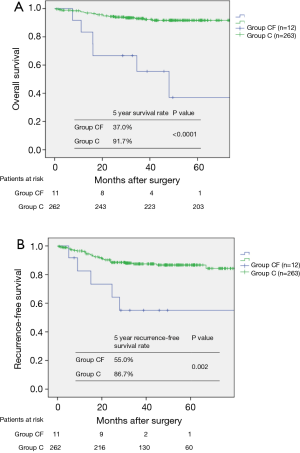
Prognostic factors
In univariate analysis, male sex, smoking habits, non-adenocarcinoma, and presence of pulmonary cavity formation were found to be associated with poor OS (P=0.008, P=0.001, P<0.0001, and P<0.0001, respectively). Multivariate analysis demonstrated that smoking habits, non-adenocarcinoma, and pulmonary cavity formation were independent prognostic factors predicting poor survival (P=0.043, P=0.004 and P<0.0001, respectively; Table 2).
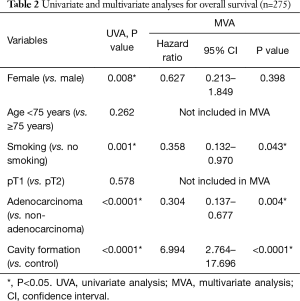
Full table
Discussion
The relative risk of lung cancer development in the wall of the bullous lung disease is higher among patients with bullous disease. Lung cancer arising from the wall of a preexisting pulmonary cystic airspace has been reported since the 1940s, but this appearance and clinical features have been described as uncommon (7). The incidence of lung cancer adjoining emphysematous bullae is reportedly 7–29% (3,14-16). Evidence suggests that emphysema is an independent risk factor for lung cancer because it increases the likelihood of lung cancer by 4- to 5-fold, and it may be a contributing factor to the development of malignancy (17). Our study also revealed that the incidence of primary lung cancer adjoining pulmonary cavity formation was 4.4% among surgical patients with even pathological stage I early primary lung cancer. In addition, male sex and smoking habits were more frequently noted in the pulmonary cavity formation group than in the control group, but no difference in the histologic type was demonstrated between these groups.
Although the mechanism of carcinogenesis adjoining pulmonary bullous disease remains uncertain, numerous theories have been proposed. First, a hypothesis of scar cancer may support an acquired etiology. Smoking is a common causative factor for both bullae and lung cancer. In our study, a higher rate of smokers was observed in group CF than in group C. Second, pulmonary cavity formations are made up of cystic air space and conducting bronchioles. Attenuated and compressed parenchyma and connective tissue make up the wall of bullae. Limited air flow in this area may cause deposition of microorganisms on the walls of pulmonary cavity formation such as bullae. Consequently, repeated infections can occur. Repeated inflammatory processes may cause formation of a fibrous scar around bullae, resulting in accumulation of carcinogens in bullae. Third, impaired ventilation of bullae may facilitate the deposition of several carcinogens in them, which causes a metaplastic transformation of the inner lining of epithelial cells. These accumulated carcinogens may stimulate the aggressive behavior of lung carcinoma arising in emphysematous bullae.
Hanaoka et al. reported that lung cancer patients with pulmonary bullous disease have a poor prognosis because their disease is advanced at the time of detection and because the tumor is aggressive in nature (15). Because the tumor cells in the present study were poorly differentiated, the histological type was large cell carcinoma. Whereas, in our study, the survival curve was worse in group CF, mainly because of the poor prognosis of the recurrent cases. However, there were no associations among the histological type, pathological tumor factor, and poor differentiation in early-stage NSCLC.
Matsuoka et al. (18) reported that malignant and benign nodules associated with emphysema exhibited considerably more overlap features in CT images than did the nodules in non-emphysematous lungs. This may cause doctor’s delay and poor prognosis. Considering these aggressive characteristics of lung carcinoma arising from emphysematous bullae, an early detection of lung tumors in patients with emphysematous bullae is preferred. Recently, interesting results were reported by the International Early Lung Cancer Action Program collaboration using CT screening to ascertain the frequency of isolated cystic airspaces associated with carcinoma (7). In all the cases detected at the annual repeat screening, CT images showed that the initial cystic airspace had a thin wall (median diameter, 1.0 mm) that subsequently became thicker; eventually, the nodule emerged, and a diagnosis of lung cancer was made. Consequently, radiologists interpreting the chest CT images of patients at risk for lung cancer should pay a careful attention to the walls of discrete airspaces because progressive wall thickening over time may represent lung cancer. We also encountered a similar case in our study (Figure 2).
This study has some limitations. The main limitations are the retrospective design, small sample size, and a single-institutional nature of this study. Secondary limitations are persistence of treatment bias and time-to-detection bias that may have influenced the worse prognostic outcomes in patients with early-stage NSCLC. Further prospective, multi-institutional investigations and substantial clinical studies are warranted for the detailed evaluation of survival correlations between pulmonary cavity formation and early-stage lung cancer.
Conclusions
We retrospectively reviewed 275 consecutive patients who underwent curative resection for pathological stage I NSCLC to investigate the impact of pulmonary cavity formation on survival outcomes. The characteristics of primary lung cancer with worse prognosis were as follows: (I) male sex; (II) smoking habits and (III) pulmonary cavity formation. Our results suggest that patients with early-stage NSCLC adjoining pulmonary cavity formation have an increased risk of poor OS and RFS after curative surgical resection.
Acknowledgements
Medical English writing assistance was provided by Crimson Interactive Pvt. Ltd. The authors are fully responsible for the content and editorial decisions for this manuscript.
Funding: This study was supported by a Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science (24592104), Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the St Marianna University School of Medicine (No. 2233). The requirement for informed consent from the patients was waived because of the retrospective study design.
References
- Bass HE, Singer E. Co-existing lobar adenocarcinoma and cystic disease of the lung. Ann Intern Med 1951;34:498-507. [Crossref] [PubMed]
- Goldstein MJ, Snider GL, Liberson M, et al. Bronchogenic carcinoma and giant bullous disease. Am Rev Respir Dis 1968;97:1062-70. [PubMed]
- Stoloff IL, Kanofsky P, Magilner L. The risk of lung cancer in males with bullous disease of the lung. Arch Environ Health 1971;22:163-7. [Crossref] [PubMed]
- Tsutsui M, Araki Y, Shirakusa T, et al. Characteristic radiographic features of pulmonary carcinoma associated with large bulla. Ann Thorac Surg 1988;46:679-83. [Crossref] [PubMed]
- Venuta F, Rendina EA, Pescarmona EO, et al. Occult lung cancer in patients with bullous emphysema. Thorax 1997;52:289-90. [Crossref] [PubMed]
- Kaneda M, Tarukawa T, Watanabe F, et al. Clinical features of primary lung cancer adjoining pulmonary bulla. Interact Cardiovasc Thorac Surg 2010;10:940-4. [Crossref] [PubMed]
- Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol 2012;199:781-6. [Crossref] [PubMed]
- Fishman AP. Fishman’s pulmonary diseases and disorders. 3rd edition. New York: McGraw-Hill, 1998.
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C. editors. TNM Classification of Malignant Tumours. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell, 2009.
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press, 2004.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-e399S.
- Ogawa D, Shiota Y, Marukawa M, et al. Lung cancer associated with pulmonary bulla. case report and review of literature. Respiration 1999;66:555-8. [Crossref] [PubMed]
- Hanaoka N, Tanaka F, Otake Y, et al. Primary lung carcinoma arising from emphysematous bullae. Lung Cancer 2002;38:185-91. [Crossref] [PubMed]
- Hirai S, Hamanaka Y, Mitsui N, et al. Primary lung cancer arising from the wall of a giant bulla. Ann Thorac Cardiovasc Surg 2005;11:109-13. [PubMed]
- de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932-8. [Crossref] [PubMed]
- Matsuoka S, Kurihara Y, Yagihashi K, et al. Peripheral solitary pulmonary nodule: CT findings in patients with pulmonary emphysema. Radiology 2005;235:266-73. [Crossref] [PubMed]


