Should cT2N0M0 be managed as a localized or locally advanced esophageal carcinoma?
Esophageal carcinoma (EC) comprises two well-defined histotypes, such as squamous cell carcinoma (SCC) and adenocarcinoma (AC), which are different each other in terms of etiology, epidemiology, prognosis and response to therapy (1). Unfortunately most studies about EC and even gastric cancer (GC) included indifferently SCC and AC, therefore making difficult to correlate the outcome to the specific histotype (2-6).
As reported in the American Joint Committee on Cancer (AJCC) 7th edition, the pathologic stage of EC comprises T1b (which invades submucosa), T2 (muscularis propria), T3 (adventitia) and T4 (adjacent structures). The N0 is correlated with stages up to the IIA and N+ regards stage IIB onwards. The T3–4 anyN and anyT N+ (IIA to IIIC stages) are defined as locally advanced and the T1–2 N0 (IA and IB stages) as localized.
The global prognosis of patients with EC remains poor, with 18% all-stages 5-year survival rate, 41% for localized and 23% for locally advanced (7). Prognosis is poorer for locally advanced SCC compared with AC (8).
While preoperative chemoradiation (CRT) (5) and perioperative chemotherapy (CHT) (6,9) compared with surgery alone have been demonstrated effective in locally advanced EC it is unclear if CHT or CRT are superior to surgery alone in localized EC. If on one hand respective surgery was always considered the first choice for the localized clinical stages on the other hand it should be taken in mind that the clinical stage could not correspond with the same pathologic stage after upfront surgery. This is particularly true for the cT2N0M0 (10). Of course the risk of clinical under-staging is directly correlated with the type of the staging work-up. For patients’ candidate to esophagectomy it is now recommended that the staging should include a neck-Chest-Abdomen CT-scan, an endoscopic ultrasound (EUS) and an FDG-PET/CT (11). However, it has been described that even with a staging including EUS and FDG-PET/CT, cN0 became pN+ after an upfront esophagectomy in 39% to 55% of cT2N0 patients (10,12,13).
On this basis localized EC were enrolled in the chemoradiotherapy for oesophageal cancer followed by surgery study (CROSS) trial (5), which demonstrated that induction CRT following by esophagectomy was superior to upfront esophagectomy. However the vast majority of tumors included in the CROSS trial were clinically locally advanced (around 80% cT3 and around 65% cN1). Furthermore despite a detailed clinical and pathological nodal assessment (cN0 31% and pN+ 75% in the upfront surgery arm) no data about the up-staging related to the cT2N0M0 were reported. The tumor population of CROSS trial was quite heterogeneous with about 60% low-third and about 15% middle third EC as the main sub-groups; moreover 75% of the whole population were AC. The Authors concluded that preoperative CRT improved survival among patients with potentially curable EC or esophagogastric-junction cancer, therefore including the cT2N0M0 as well.
The precise hypothesis that the neo-adjuvant treatment could be effective in clinically localized EC was the basis of the phase III trial FFCD 9901, comparing pre-operative CRT with upfront surgery in 195 patients with stage I–II EC, based on the AJCC TNM 5th edition (14). All tumors were thoracic EC, 90% below carina; 70% were SCC. Patients were enrolled on the basis of the 5th edition of the AJCC TNM Classification (15). Staging was based on Chest-Abdomen CT and EUS; 53% of patients had a stage IIA (cT2N0M0). No stage-migration data were reported.
This study showed no survival benefit in the CRT arm, although the tri-modal approach produced a significant advantage in locoregional recurrence and recurrent disease rate. A well scheduled pathological staging was reported for patients undergoing resection, with almost 40% of pathological stage III in the surgery arm, and about 12% in the CRT arm. Despite the Authors stated that upfront esophagectomy should be the first choice for localized EC, no definitive conclusions can be drawn for cT2N0M0, since a related sub-group analysis was not conducted.
Two previous trials had attempted to investigate neo-adjuvant CRT compared with surgery alone in early-stage EC (16,17). However the power of both were limited by suboptimal staging procedures, a non-standardized surgical approach and outdated neo-adjuvant treatment regimens. In the study by Le Prise et al., clinical staging using CT scan was not performed routinely, whereas EUS and FDG-PET/CT were not performed at all; the histologic analysis of patients treated solely with surgery revealed that more than half of patients had a locally advanced rather than an early-stage disease. In the study by Bosset et al., no survival benefit was shown, with significantly more post-operative deaths after neo-adjuvant CRT.
A major solid answer should derive from a randomized prospective trial in the specific setting of cT2N0M0 that has never been conducted so far. Currently we have only data from retrospective studies showing contrasting results.
In this journal, Markar et al. reported findings from a retrospective multi-center European study, the FREGAT, about 355 patients with cT2N0M0 EC, extrapolated by a website referring to 30 French-speaking European centres (18). All patients had been staged with CT scan and upper digestive US. Their findings were in line with FFCD 9901 trial and failed to demonstrate a benefit of induction therapy in terms of survival compared to upfront surgery, regardless of pathological TNM. The only advantage was described for the pathological T and N down staging. Additionally, despite retrospectively analyzed, the FREGAT study showed that the benefit of surgery was preserved independently from histotype (SCC or AC) and type of neo-adjuvant treatment (CRT or CHT). However some weaknesses of this study should be highlighted. Firstly, almost 18% of patients received an adjuvant therapy, which could have partially affected the outcome; secondly, the type of adjuvant treatment administered was not specified, if RT or CHT or both.
The aforementioned data (prospective and retrospective) seem to indicate that induction therapy of cT2N0M0 EC is not effective, even though the answer to the question if an upfront surgery or induction therapy should be preferred in cT2N0M0 EC is still pending. Probably the right question should be which cT2N0M0 patients deserve an induction therapy and which not? A recently published retrospective study addressed this issue, reporting a large retrospective series of cT2N0M0 EC, with 1,785 selected patients; 52% underwent upfront esophagectomy and 48% induction therapy followed by esophagectomy. This series represents 9% of all esophagectomies in M0 patients from 2006 to 2012 in the national cancer database (NCDB). Among the up-staged patients, which were 46% of the total, those receiving induction therapies had a significantly better OS (10). Importantly, the cT2N0 patients receiving upfront esophagectomy were significantly more likely to be pathologically up-staged versus induction therapy patients. Additional findings revealed that cT2N0 patients receiving upfront esophagectomy were significantly more likely to have a higher tumor grade and a higher rate of lymphovascular invasion, hence identifying a subgroup of patients at increased risk of pathological up-staging. Interestingly, up-staged patients from upfront surgery (who were almost 50%) who did not receive adjuvant therapy had a detrimental survival, although non-statistically significant, compared with those who did not receive an adjuvant therapy. Therefore, extrapolating the results of this study an induction therapy (CHT or CRT) could be effective in a subset of patients at increased risk for pathologic stage-migration, such as those with high tumor grade and lymphovascular invasion.
Therefore more in general in cT2N0M0 EC a CRT should be proposed according to the phase III CROSS trial, whereas no induction therapy should be considered according to the phase III FFCD 9901 trial. Unfortunately, neither CROSS nor FFCD 9901 were specifically focused on cT2N0M0. The only studies precisely addressing the cT2N0M0 EC, such as the FREGAT and Samson’s, were retrospective and they reported controversial conclusions. The FREGAT study and main randomized trials is shown in Tables 1,2.
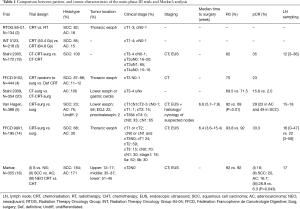
Full table
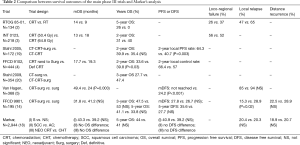
Full table
Therefore the debate about cT2N0M0 EC still remains freezed into the watershed, since on one hand an upfront esophagectomy could be a putative undertreatment in this clinically under staged tumor population and on the other hand CRT could be an overtreatment for those around 50% of real cT2N0.
Moreover, as we do not have any evidence about adjuvant CRT in EC, choosing upfront esophagectomy as first choice could deprive those 50% of pN+ patients of receiving a proved effective CRT treatment in the preoperative setting.
Not least post-operative morbidity and mortality should represent a further factor for choosing between upfront surgery and induction therapy. In the FREGAT study, no significant difference in terms of in-hospital mortality and morbidity between surgery and CRT groups was observed, in line with the CROSS trial and FFCD 9901 trial, with the only exception of in-hospital mortality, slightly higher in the CRT subgroup, in this latter study.
In conclusion, given that stage-migration remains an issue even with the best staging procedures it is plausible thinking about a role of a neo-/adjuvant therapy in cT2N0M0 EC (21). Furthermore we think that on the basis of the current evidence it is probable that neo-adjuvant therapy is effective in some cases. Keeping in mind this hypothesis, clinicians should perform the best staging, with neck-Chest-Abdomen CT-scan + EUS + FDG-PET-CT, and they should try to obtain pathologic information about histotype, tumor grade and lymph-vascular invasion. Each case should be discussed within a multidisciplinary team, including surgeon, medical oncologist, endoscopist and radiotherapist, also considering the site of the tumor, the surgery invasiveness and clinical conditions of the patient. For patients clinically fit a neo-adjuvant CRT, CROSS trial-like, could be considered. Although it is not clear if cN+ has the same prognostic impact than cN0/pN+ and if a neoadjuvant CHT or CRT might improve prognosis in this setting, we think that this is better than discussing an adjuvant CHT or CRT after an upfront esophagectomy of upstaged EC.
It was not demonstrated if SCC and AC should be managed at the same way. At least for locally advanced low third esophageal AC recent evidence in favor of perioperative CHT (9) should be considered; similarly for locally advanced upper and mid third esophagus SCC even definitive CRT should be considered (19).
Further investigation about identification of predictive factors of high-risk upstaging tumors will be strongly encouraged. The answer to this dilemma is more likely to be found in a hypothetical study, which would compare the long term outcome of pathological T2N0 retrospectively assessed for upfront surgery versus preoperative approach, and separately analyzed according to histotype, baseline homogeneous clinical staging, type of induction therapy and adjuvant treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Castro C, Bosetti C, Malvezzi M, et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980-2011) and predictions to 2015. Ann Oncol 2014;25:283-90. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg 2001;234:360-7. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Samson P, Puri V, Robinson C, et al. Clinical T2N0 Esophageal Cancer: Identifying Pretreatment Characteristics Associated With Pathologic Upstaging and the Potential Role for Induction Therapy. Ann Thorac Surg 2016;101:2102-11. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-v57. [Crossref] [PubMed]
- Crabtree TD, Yacoub WN, Puri V, et al. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg 2011;91:1509-15. [Crossref] [PubMed]
- Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg 2011;92:491-6. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Sobin LH, Fleming ID. TNM Classification of Malignant Tumors. 5 ed. New York: John Wiley and Sons, 1997.
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [Crossref] [PubMed]
- Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994;73:1779-84. [Crossref] [PubMed]
- Markar SR, Gronnier C, Pasquer A, et al. Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: results from a retrospective multi-center European study. Eur J Cancer 2016;56:59-68. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Speicher PJ, Ganapathi AM, Englum BR, et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J Thorac Oncol 2014;9:1195-201. [Crossref] [PubMed]


