Age and blood transfusion: relationship and prognostic implications in cardiac surgery
Introduction
In developed countries, octogenarians represent the fastest-growing segment of the population. Over 40% of these individuals manifest cardiovascular disease, frequently in an advanced state, requiring cardiac surgical procedures. Higher rates of comorbid risk factors are frequently encountered in this patients’ subset, which may result in more frequent and severe complications, and inferior survival rates. Aortic calcification, stiff vessels, diminished cardiovascular response to exercise, and intolerance of anaemia are some known markers of risk. More, performance and physiologic reserve of the respiratory, renal, and nervous systems are inversely related to ageing. Despite significant improvements, mortality, morbidity, and resource utilization in this high-risk group remain substantial (1-4). In particular, elderly patients presenting for cardiac surgery are often anaemic and require transfusion of larger amounts of perioperative red blood cells (RBC). Clinical studies emphasize the paradox that both anaemia and transfusion are associated with organ injury and worse outcomes after surgery (5,6). Acute kidney injury (AKI) is a serious and common complication of cardiac surgery. Even though the underlying pathophysiological mechanism has not been fully elucidated, numerous observational studies have shown that perioperative RBC transfusion and AKI are closely associated with each other. Indeed RBC transfusion proved a risk factor for AKI and, on the other side, AKI emerged as a major determinant of RBC need. Moreover, a growing body of evidence indicates that anaemic patients are more susceptible to transfusion-related AKI than non-anaemic patients (7). Nevertheless, data about the outcomes of transfusion in elderly patients are scarce and contradictory (8-10). There is scant evidence that RBC need is higher in this patients’ subset. More, it is still unclear whether transfusion is an independent risk factor for both post-operative morbidity and mortality or a mere marker of risk. There is also evidence that, despite pre- and post-operative anemia, per se, may portend adverse outcomes; associated co-morbidities emerged as the main determinants of hospital survival. Therefore, this study aimed to investigate the relationship between age and blood transfusion. The study explored also the relationship of RBC transfusions and Age with AKI and survival.
Methods
Study setting and patient sample
The study was conducted at the Division of Cardiac Surgery of the “Casa di Cura Montevergine”, a private hospital in Mercogliano, Avellino, Italy. At this institution, nearly 700 patients undergo cardiac surgery annually and are admitted to a dedicated 8-bed postoperative intensive care unit. Information from these patients is collected on a daily basis, using standardized case report forms; all clinical peri-operative data are entered into a computerized database. Out of 1,984 consecutive patients referred for cardiac surgery, between January 2013 and December 2015, 1,765 patients (age: 67.6±10.3 years; octogenarians: 176; female: 33.1%; redo: 6.2%; urgent/emergent: 12.9%; isolated CABG: 40.1%; isolated valve procedures: 30.4%; combined: 26.7%), who received no preoperative transfusion and underwent on-pump procedures, constituted the study sample.
Study design and aims
The aim of this observational retrospective study, on prospectively collected data, was to determine whether age is an independent predictor of the need for transfusion. Indeed, it aimed to untangle whether age per se may determine transfusional need independently from age related co-morbidities and the pattern of perioperative course and care. The study aimed also to identify the relationship between RBC transfusion and both survival and AKI, and any interaction with age. In this respect, the study aimed to disclose whether survival and AKI are related to the need and the amount of transfusions and whether age plays an independent role on these outcomes. The Clinica Montevergine Ethics and Research Committee, which waived the need for informed consent, approved the research protocol (Clinica Montevergine Ethics and Research Committee protocol number 27/2015) that is fully in accordance with the Helsinki Declaration as revised in 2013.
Surgical and clinical care
The same five senior surgeons performed all procedures throughout the study period. Details of the surgical strategy and postoperative care are reported extensively elsewhere (11). Aprotinin was never used as a preventative for bleeding, since its use is not authorized in Italy; tranexamic acid was given preoperatively to patients on dual antiplatelet therapy. Heparinization was managed by monitoring both heparin blood levels and activated coagulation time (ACT) throughout the operation. The heparin loading dose was 300–400 IU/kg with a target ACT of at least 400 seconds. An intraoperative autologous blood salvage method was used in every patient with preoperative anemia or receiving ongoing dual antiplatelet therapy. A specific perioperative transfusion algorithm was applied: patients received two packed red cells units before cardiopulmonary bypass (CPB) whenever the preoperative hematocrit value was below 30%, and they received two or more packed red cells units during CPB in case of excessive hemodilution (hematocrit value below 22%). After CPB, the patients received packed red cells in order to maintain a hematocrit value higher than 25%. This target value was raised to higher values according to the clinical condition, and namely to the hemodynamic status, the need for inotropic support, and oxygen delivery. Fresh frozen plasma was not used prior to reaching the ICU. Platelets were usually not transfused, except in patients reaching the operating room under full dose of ticlopidine or clopidogrel and showing severe postoperative bleeding. This protocol complied with those adopted in major centers carrying out these procedures and, to a lesser extent, with recently published guidelines (12,13).
Clinical outcomes
All definitions were established as part of the original study design. The gender-based definition of anemia (12.0 g/dL in women and 13.0 g/dL in men) complied with statements by the World Health Organization (WHO). The WHO grading scale for anemia severity was adopted. The incidence of cardiac surgery associated AKI (CSA-AKI) according to the risk, injury, failure, loss, end-stage (RIFLE) criteria were investigated (14). The change in kidney function was based on plasma creatinine concentration and defined as the difference between baseline concentration and the highest concentration during the stay in ICU. The preoperative glomerular filtration rate (GFR) and nadir GFR during ICU stay were calculated with the Modification of Diet in Renal Disease equation: estimated GFR = 186 × [plasma creatinine level (in mg/dL)] − 1.154 × [age (in years)] − 0.203. For women, the product of this equation was multiplied by the correction factor of 0.742 (15). The National Kidney Foundation Classification staging system was adopted (15). AKI with eGFR loss >50% was the target outcome. Mortality was defined as any death occurring within 30 days after surgery.
Statistical analysis
A complex, multistage statistical approach was developed. In order to depict the impact of aging on cardiac surgery practice (pattern of referrals, surgical approach and crude outcomes), the overall study population was divided into two groups according to age (<80 and >80) as generally accepted in cardiac surgical literature (7-9). Meaningful differences were evaluated through univariate analysis (using the χ2 statistic for categorical variables and the t-test or Wilcoxon rank-sum for continuous variables). In order to address the predictors of transfusion the following approach was implemented. Univariate analysis was adopted to identify significant preoperative and intraoperative factors associated with transfusion requirements in the general surgical population, using as dependent variable a dichotomous variable reflecting the prevalence of transfused patients. Transfusion practice to which risk factors were correlated was defined as transfusion of one or more units of red cells. Multivariate logistic regression analysis with forward selection was adopted to identify independent predictors of transfusion. Only significant variables at univariate analysis (P≤0.05) were included in the model.
The need for RBC transfusion in cardiac surgery derives from a complex interplay between preoperative factors (such as anemia and physiological tolerance to anemia, status of the procedure, preoperative medication) intraoperative factors (extent and complexity of surgery, technical issues and complications) as well as, postoperative course (bleeding, reoperation, major complications, enhanced invasiveness of the process of care). In order to overcome the inherent limits of such a pattern and more accurately describe the relationship between age and transfusion, a propensity score for the likelihood to receive RBC transfusion was calculated for each patient using multivariate logistic regression analysis, with the single-step entry method. As generally recommended, the authors focused on their clinical experience and literature data for selection of relevant variables. Variables included in the model were: sex, age (continuous variable), body mass index (BMI), body surface area (BSA), preoperative hemoglobin, preoperative eGFR, preoperative anemia, left ventricular ejection fraction, pulmonary hypertension, preoperative antiplatelets or anticoagulant therapy, EuroSCORE, redo-procedure, surgical priority, surgery on the thoracic aorta, length of extra corporeal circulation, length of aortic clamping, need for deep hypothermic circulatory arrest, need for perioperative IABP, peak of cardiac troponin release, surgical revision for bleeding, reoperation for any cause, reintubation, neurological dysfunction, and need for renal replacement therapy. Redundancy in variable selection, as recommended, aimed to enhance the performance of the model. A good predictive performance (area under the receiving-operator curve =0.81, 95% CI: 0.79–0.83) was demonstrated for the model. This propensity score ultimately incorporates all the triggers that might determine transfusion in this cardiac surgical population. This method complies with current recommendations for statistical analysis in biomedical studies and follows that originally employed by the same group in J Thorac Cardiovasc Surg (16), which is a F1000Prime recommended article, cited several times in inherent research works and even adopted in current international specialty guidelines on this complex topic. A logistic estimation curve was then developed to investigate for the interaction (R-square) between this propensity score and age (as a continuous variable) in order to address whether age per se may independently predict transfusion. Finally, age (as a continuous variable), number of RBC transfusions and the balancing score for transfusions were forced into multivariate logistic models (forward selection) for other study outcomes (AKI and survival). In other words, the study did not aim to develop a multivariate model for all possible determinants of AKI and mortality, which is actually obsolete/futile data in the cardiac surgical literature since the development of the preoperative cardiac surgical scoring systems, but closely focused on study aims. Data are expressed as mean ± SD [or median and interquartile range (IQR) when appropriate] for continuous variables and as percentages for categorical variables. All statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written. The study protocol and the overall data report comply with the STROBE statement.
Results
Study sample features
RBC transfusion was performed in 41% of the entire cohort. Patients ≥80 years of age underwent transfusion more often than patients <80 years (52.9% vs. 39.7%; P<0.001) with a net 1.3-fold increase in the relative risk for transfusion. The median number of RBC units transfused was 0; (IQR 0–1); in adults it was 0; (IQR 0–1) while in octogenarians it was 1; (IQR 0–1.75), which was a statistically significant increase (P=0.003). Study sample features and outcomes are reported in Tables 1-3. Transfused patients, irrespective of age, had a more complex preoperative profile. Transfused elderly patients displayed lower preoperative eGFR, a higher incidence of anemia, and had a lower BMI. No difference in the surgical priority or extent of surgery was seen between the transfused subsets. The mean length of ICU stay was 2.76±2.4 days; octogenarians needed longer high intensity assistance (3.1±2.9 vs. 2.71±2.36; P=0.018). AKI and mortality rates were significantly higher in the transfused subsets, irrespective of age, although elderly transfused patients displayed significantly higher mortality rates. The rate of transfusion according to the decade of patients’ age was as follows: 20% in patients aged <30; 28.6% in patients aged 30 to 40; 33.3% in patients aged 40 to 50; 31.4% in patients aged 50 to 60; 36.6% in patients aged 60 to 70; 48.4% in patients aged 70 to 80; and 52.2% in patients older than 80 (P<0.0001). The rate of transfusion according to preoperative anemia severity was as follows: 46.2% in mild anemia; 68.5% in moderate anemia and 100% in severe anemia (P<0.0001). The rate of transfusion according to preoperative kidney function as described by CKD stages was as follows: 31.6% in CKD 0 and 1; 36.1% in CKD 2; 57.8% in CKD 3a and 78.4% in CKD 3B (P<0.0001).
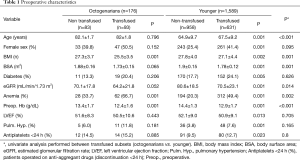
Full table
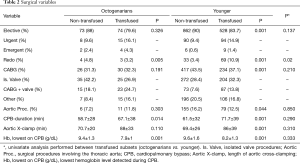
Full table
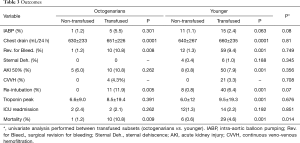
Full table
Predictors of transfusion
Univariate predictors of transfusion were as follows: age, female sex, BSA, BMI, NYHA class, eGFR, preoperative hemoglobin, pulmonary hypertension, EuroSCORE, antiplatelets discontinued within 24 hours before surgery (patients operated on antiaggregants), endocarditis, emergent surgery, redo procedure, extent of surgery, length of extracorporeal circulation, and length of aortic cross-clamping. Independent predictors of transfusion were: preoperative hemoglobin (β: −0.5; OR: 0.65; 95% CI: 0.6–0.7, P<0.0001); baseline eGFR (β: −0.01; OR: 0.98; 95% CI: 0.98–0.99, P<0.001); BSA (β: −1.05; OR: 0.35; 95% CI: 0.19–0.64, P=0.001); antiplatelet drugs within 24 hours preoperatively (β: 1.4; OR: 4; 95% CI: 1.4–11.6, P=0.01); priority (β: 0.52; OR: 1.7; 95% CI: 1.2–2.4, P=0.005); redo procedure (β: 0.78; OR: 2.2; 95% CI: 1.3–3.6, P=0.003); isolated valve procedure (β: −0.66; OR: 0.51; 95% CI: 0.33–0.88, P=0.004) ; CPB time (β: 0.01; OR: 1.01; 95% CI: 1.008–1.015, P<0.0001) .
The propensity score for transfusion and its correlation with age
As reported above, a propensity score for transfusion was developed in order to adjust for confounders. It included preoperative, intraoperative, as well as postoperative features related to patient blood management. A lack of interaction between this score and age (R-square =0.095) emerged.
Independent predictors of study outcomes
The relationship between age, propensity score for transfusion, number of transfusions, and AKI was explored by logistic regression analysis. Age (β: 0.04; OR: 1.04; 95% CI: 1.01–1.08, P=0.010); Propensity Score (β: 2.7; OR: 14.7; 95% CI: 4.5–4.8, P<0.0001) and number of blood transfusion (β: 0.27; OR: 1.3; 95% CI: 1.18–1.44; P<0.0001) proved independent predictors of AKI.
The relationship between age, propensity score for transfusion, number of transfusions, and mortality was explored by logistic regression. Age (β: 0.6; OR: 1.06; 95% CI: 1.01–1.1, P=0.015), propensity Score (β: 6.7; OR: 792; 95% CI: 88.9–7060, P<0.0001), and number of blood transfusion (β: 0.14; OR: 1.15; 95% CI: 1.05–1.25; P<0.0001) emerged as independent predictors of mortality.
Discussion
This clinical series provides a valuable outlook on the current status of elderly patients undergoing cardiac surgery and the associated outcomes with a particular focus on the prognostic implications of patient blood management. Critically ill elderly patients are becoming an increasingly common demographic in surgical theatres. Indeed, in this cohort, they represented nearly 10% of patients, one of the highest percentages ever reported, which confirmed the temporal trends depicted in a large multicentre cohort study a decade earlier (1). Compared with prior series, the gap in postoperative mortality and morbidity rates between octogenarians and younger patients appears to be narrower (2,3). Notably, elderly patients without any significant comorbidity profile experienced an in-hospital mortality rate as low as 1.2%. More, ICU recidivism has long been proposed as a marker of quality of care. Though overall rates of ICU readmissions were within reported ranges (1.7%), age did not exert any significant impact on recidivism rates, a finding that is somewhat at odds with contemporary reports on larger series (4). Increased experience in patient selection, surgical skill, and perioperative management along with the peculiar setting, i.e., a non-teaching, private practice may explain these results. Data on the interaction between transfusion and age are rare and contradictory (8-10). The present series revealed that, even though a significantly higher amount of transfusion is needed in elderly patients undergoing cardiac surgical procedures, age per se proved not to be a determinant of the need for RBC. Indeed predictors of transfusion encompass preoperative haemoglobin, baseline eGFR, antiplatelet medication (ticlopidine and clopidogrel) up to 24 hours prior to surgery, BSA, surgical priority, redo procedure, extent of surgery, and the length of the CPB. Notably, as far as the extent of surgery is concerned, isolated vale procedures, which are straightforward and less invasive than myocardial revascularization and aortic procedures proved protective, as showed by the β value at multivariate analysis. Such results are consistent with our previous study (17). Taken together, these data underscore that optimization of patient management strongly relies on careful patient selection, enhancement of preoperative health status, improvement of extracorporeal circulation equipment and management, along with meticulous surgical planning and execution. In the study described here, the interaction between the propensity score and age was poor and predictors of hospital outcomes were similar in both the young and the old. This confirmed a concept derived from a major series reported a decade ago, and, ultimately, testifies of the relentlessly complex comorbid profile of the population referred for surgery, which extends well beyond aging (1).
The burden of AKI in cardiac surgery is still substantial and remains prognostically relevant. Pathophysiological mechanisms implicated in the genesis of AKI after CPB are multiple, but the synergistic role of anaemia and transfusion is a key factor (18). In this respect, data from this study, add to current knowledge, since prognosticators of AKI were both the balancing score for transfusion and transfusion itself in a clear dose-dependent relationship. The burden of AKI is relevant also to the setting of trans-catheter aortic valve implantation. AKI affects 12% to 57% of those patients and implies a 2- to 6-fold increased risk of death. The vicious circle linking anaemia, transfusion, and AKI was also highlighted in this setting, with three implications (19,20). First, CPB may not be more dangerous than trans-catheter vessel manipulation and contrast media loading in the highly vulnerable elderly, perhaps because such vulnerability arises well beyond/before intervention. Second, as previously reported: “issues with RBC transfusion and anaemia constitute work-in-progress as the field moves toward more patient-centred anaemia and blood management” (21). In this respect, even though the current evidence indicates that preoperative anaemia is still not adequately treated even in elective surgery and in the most advanced public healthcare programs, new treatment paradigms are emerging beyond the highly questionable implementation of the preoperative erythropoietin plus iron strategy (22,23). Most recently, prophylactic erythrocyte transfusion has been proposed as a means of reducing perioperative anaemia (24). Such a new approach “may allow time for iron metabolism to stabilize before the effects of surgery come into play” and optimize oxygen delivery (24). Third, anaemia should be included in new operative risk stratification models due to its prognostic implications, as suggested by Scrascia and co-workers (25).
In the present study, the logistic regression analysis revealed transfusion and the propensity score for transfusion as independent predictors of mortality. Within the obvious restraints of an observational design, which prevents the elucidation of causal relationships, and those imposed by the study aims, this study has added to the knowledge that transfusion is associated with death (5). Nevertheless, the propensity score for transfusion is also an independent predictor of survival. Such a statistical entity encompasses at least two biological features, i.e. anaemia and renal function, which are known for their inherent prognostic implications. This also includes the most dreaded complications that a patient may experience after a cardiac surgical procedure. In other words, the debate on the harmful effects of transfusion and the intrinsic vulnerability of the transfused patient remains unanswered (26).
Study overview
Several study limitations should be considered to allow thorough data interpretation. First, the single-centre setting, although guaranteeing a uniform process of care, with special emphasis on transfusion triggers, closely reflects the influence of specific standards of clinical practice and a unique patient population, which may have led to biased results that are not readily transferable to other patient populations. However, in this study, the inclusion of all consecutive patients admitted for cardiac surgery, high quality prospective data-mining, and the use of appropriate statistical processes for adjusting confounders ensure that the results are objective and transferable. The present cohort includes both high-priority procedures and massive blood transfusions that, given their peculiar risk profile, are often excluded by other studies on this topic. Inclusion of these subsets was intended to reproduce a real-world setting and has enhanced the chances of extrapolating these findings to other experiences. There is some evidence that the relationship between transfusion and adverse outcomes is affected by donor blood processing (leukodepletion) and storage duration (17). Our study lacks any information about the length of RBC storage, and institutional donor blood processing did not entail leukodepletion. AKI definition according to the “old” RIFLE criteria may deserve a final consideration. Definitions of AKI have evolved fairly rapidly in recent years, from RIFLE [2004], through AKIN [2007], to KDIGO [2012] (a merger of RIFLE and AKIN, but with less rigorous requirements for detection in those patients with chronic kidney disease). All three systems are complex and rely on non-SI units for creatinine. As reported in a recent authoritative review by Thomas ME and co-workers: “Systematic review has found that these definitions do not differ significantly in their performance” (27).
Conclusions
This series testifies to the relentless aging of the cardiac surgical population. Although perioperative management of elderly patients implies significantly more transfusion, age per se proved not to be a determinant of the need for RBC transfusions. Optimization of patient blood management strongly relies on careful patient selection, enhancement of preoperative health status, improvement of extracorporeal circulation equipment and management, along with meticulous surgical planning and execution. Hospital outcomes and predictors of hospital outcomes were similar in both the young and the old. Given the inherent vulnerability imposed by aging, development of more patient-centered anemia and blood management algorithms are warranted.
Acknowledgements
Authors are grateful to Mrs. Valeria Rocco for her meticulous editing of the main text and creating the tables.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Clinica Montevergine Ethics and Research Committee, which waived the need for informed consent, approved the research protocol (Clinica Montevergine Ethics and Research Committee protocol number 27/2015).
References
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients > or = 80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000;35:731-8. [Crossref] [PubMed]
- Scott BH, Seifert FC, Grimson R, et al. Octogenarians undergoing coronary artery bypass graft surgery: resource utilization, postoperative mortality, and morbidity. J Cardiothorac Vasc Anesth 2005;19:583-8. [Crossref] [PubMed]
- Schurr P, Boeken U, Litmathe J, et al. Predictors of postoperative complications in octogenarians undergoing cardiac surgery. Thorac Cardiovasc Surg 2010;58:200-3. [Crossref] [PubMed]
- Litwinowicz R, Bartus K, Drwila R, et al. In-hospital mortality in cardiac surgery patients after readmission to the intensive care unit: a single-center experience with 10,992 patients. J Cardiothorac Vasc Anesth 2015;29:570-5. [Crossref] [PubMed]
- Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52. [Crossref] [PubMed]
- Du Pont-Thibodeau G, Harrington K, Lacroix J. Anemia and red blood cell transfusion in critically ill cardiac patients. Ann Intensive Care 2014;4:16. [Crossref] [PubMed]
- Karkouti K, Wijeysundera DN, Yau TM, et al. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology 2011;115:523-30. [Crossref] [PubMed]
- Veenith T, Sharples L, Gerrard C, et al. Survival and length of stay following blood transfusion in octogenarians following cardiac surgery. Anaesthesia 2010;65:331-6. [Crossref] [PubMed]
- Yun JJ, Helm RE, Kramer RS, et al. Limited blood transfusion does not impact survival in octogenarians undergoing cardiac operations. Ann Thorac Surg 2012;94:2038-45. [Crossref] [PubMed]
- Carrascal Y, Maroto L, Rey J, et al. Impact of preoperative anemia on cardiac surgery in octogenarians. Interact Cardiovasc Thorac Surg 2010;10:249-55. [Crossref] [PubMed]
- De Santo LS, Bancone C, Santarpino G, et al. Microbiologically documented nosocomial infections after cardiac surgery: an 18-month prospective tertiary care centre report. Eur J Cardiothorac Surg 2008;33:666-72. [Crossref] [PubMed]
- Ranucci M, Romitti F, Isgrò G, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg 2005;80:2213-20. [Crossref] [PubMed]
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris VA, Brown JR, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944-82. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. [Crossref] [PubMed]
- De Santo L, Romano G, Della Corte A, et al. Preoperative anemia in patients undergoing coronary artery bypass grafting predicts acute kidney injury. J Thorac Cardiovasc Surg 2009;138:965-70. [Crossref] [PubMed]
- De Santo LS, Amarelli C, Della Corte A, et al. Blood transfusion after on-pump coronary artery bypass grafting: focus on modifiable risk factors. Eur J Cardiothorac Surg 2013;43:359-66. [Crossref] [PubMed]
- Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth 2012;109 Suppl 1:i29-i38. [Crossref] [PubMed]
- Nuis RJ, Rodés-Cabau J, Sinning JM, et al. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2012;5:680-8. [Crossref] [PubMed]
- Nuis RJ, Sinning JM, Rodés-Cabau J, et al. Prevalence, factors associated with, and prognostic effects of preoperative anemia on short- and long-term mortality in patients undergoing transcatheter aortic valve implantation. Circ Cardiovasc Interv 2013;6:625-34. [Crossref] [PubMed]
- Koch CG. Invited commentary. Ann Thorac Surg 2012;94:2045. [Crossref] [PubMed]
- Gombotz H, Rehak PH, Shander A, et al. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion 2014;54:2646-57. [Crossref] [PubMed]
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012;116:613-21. [Crossref] [PubMed]
- Vincent JL, Lelubre C. Preoperative transfusions to limit the deleterious effects of blood transfusions. Anesthesiology 2012;116:513-4. [Crossref] [PubMed]
- Scrascia G, Guida P, Caparrotti SM, et al. Incremental value of anemia in cardiac surgical risk prediction with the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II model. Ann Thorac Surg 2014;98:869-75. [Crossref] [PubMed]
- Dardashti A, Ederoth P, Algotsson L, et al. Blood transfusion after cardiac surgery: is it the patient or the transfusion that carries the risk? Acta Anaesthesiol Scand 2011;55:952-61. [PubMed]
- Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015;87:62-73. [Crossref] [PubMed]


