Surgery in 2013 and beyond
Introduction
Epidemiology
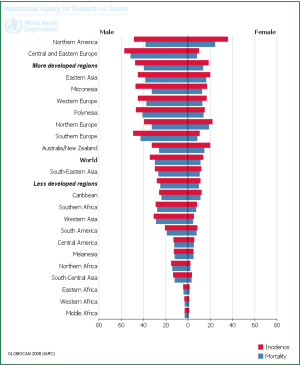
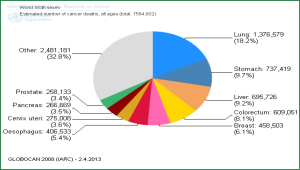
Lung cancer remains a leading cause of cancer related mortality with the WHO reporting 1,380,000 deaths from it in 2008 (Figure 1) (1). It is the most common cancer in men worldwide, fourth in women and globally is responsible for more deaths that breast and prostate cancer combined. Tobacco consumption is incriminated in 85-90% of lung cancer cases.
In Australia, lung cancer is the 5th most commonly diagnosed cancer (2). It poses a significant health burden with an incidence rate of 43.2 cases per 100,000 people. Lung cancer is also the most common cause of cancer death accounting for 18.9% of all cancer deaths (2). Survival rates overall are poor, but the trend is improving with time, being 8.7% for 1982-1987 and increasing to 14.1% for 2006-2010 (3).
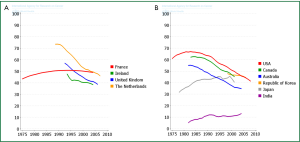
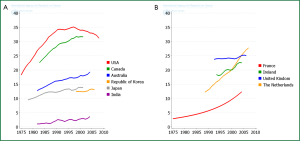
Worldwide the epidemiology varies due to socio-economic factors. In more developed countries the incidence is falling in men but is still rising in women (1) largely due to successful efforts at tobacco control and smoking cessation efforts (Figures 2,3,4). Peak incidence in more developed countries is now in the 8th decade. In less developed countries the lung cancer epidemic is in an earlier phase. Incidence is low but rising rapidly in men and women, and peak incidence occurs 2 decades earlier.
Tumour pathology—non-small cell lung cancer (NSCLC)
Recent developments in molecular profiling have accentuated the role of the pathologist within the multi-disciplinary team. No longer is it appropriate for the pathologist to determine simply whether a specimen is either small cell or NSCLC. In more developed countries there has been a marked change in the histopathologic profile of non-small cell carcinoma, squamous cell carcinoma no longer being the most common cell type. Recent trends show a significant increase in adenocarcinoma and a shift towards more peripheral squamous cell tumours (4).
Additionally the subclassification of adenocarcinoma into recognizable histologic groups has important prognostic and therapeutic implications. The pathologist will now routinely be required to perform an ever increasing array of tumour genomic assays as many unique tumour mutations and amplifications have been identified that allow targeted therapeutic options. The testing for these mutations is becoming cheaper and in many centres it is now routine to have results on the EGFR and ALK mutation status of all adenocarcinomas. These can be tested on fewer cells, in some cases only 100 cells may be required (5).
Koudelakova et al. recently reviewed the clinically relevant driver mutations (6). Epidermal growth factor receptor (EGFR) gene mutations occur in 10-30% of patients with non-small cell lung cancer (6,7). Tyrosine kinase inhibitors (TKI) have been demonstrated to show responses in 70-80% of patients with this mutation (6,7). Erlotinib and gefitinib have higher response rates and longer progression free survival compared to chemotherapy. Response rates in EGFR negative patients are low. Adenocarcinomas, females and non-smokers have been shown to respond better. Current recommendations are that all newly diagnosed patients with advanced NSCLC be tested, and if positive, should be commenced on a TKI.
The anaplastic lymphoma kinase (ALK) oncogene has been found in 5% of patients, increasing to as high as 20% in light or non-smokers (8). Crizotinib, an ALK TKI, has been shown to be effective and phase III trials are ongoing. It is recommended that this mutation also be tested for.
The thoracic surgeon needs to be well aware of these developments not only to counsel the patient about the implications of such tests in resected specimens but to be fully involved within the multi-disciplinary team during discussions for “more tissue” (9). In patients with advanced metastatic disease, it is imperative that the surgeon brings to the table a realistic assessment of the risk/benefit of the proposed procedure, has knowledge of the chances of a positive result and is fully aware how much tissue is required before embarking on further invasive procedures.
Surgery—where are we now?
Surgical management is the standard of care for stage I and II in patients who are medically fit even though there are not randomised controlled trials of surgery versus other therapy in these patients (10,11). Expected 5-year survival figures are 60-80% for stage I and 40-60% for stage II. In a meta-analysis on the role of surgery, Wright et al. analysed trials of surgery against no treatment or non-surgical treatment, concluding that they could neither support nor discount the survival benefit of surgery but that “a little surgery was better than none” (12). There also is a role for surgery in selected stage IIIA cases, usually in a multi-modality setting, and even highly selected cases of stage IIIB and IV cases surgery may merit consideration.
Staging for lung cancer currently follows the TNM classification in its 7th edition and the reader is referred to the IALSC Staging Manual in Thoracic Oncology (13). There has been a logical evolution in trying to select those patients who will benefit from surgical resection and to exclude those in whom surgery will offer no assistance, the so called ‘futile thoracotomy’. The dominant focus is the status of the mediastinal lymph nodes. After the introduction of invasive mediastinal assessment by Daniels [1949], Carlens [1959] and McNeill and Chamberlain [1966], these became the traditional preoperative modes of assessment for the next 40 years (14-16). Accuracy was quite high and these techniques became well established. Cervical mediastinoscopy however, is difficult to teach, and in inexperienced hands a procedure with morbidity and mortality rates. In general, there is strong evidence to suggest that it has been underutilized particularly in low volume centres as outlined in the review by Little et al. in 2005 (17). Video-assisted mediastinoscopy has been a considerable advance providing improved visualization especially for training purposes.
Over the last 30 years Computed Tomography (CT), has come to occupy a central role in assessing the intrathoracic extent of disease and occasionally detects occult distant disease. Assessment of the T component of stage is assisted by CT scan but all surgeons will be aware of the uncertainties in deciding resectability from the CT scan. MRI is usually reserved for apical sulcus lesions and sometimes T4 tumours in which the ability to reconstruct in oblique axes may be advantageous. Nodal assessment by CT scan has limited accuracy particularly with nodes <15 mm in short axis dimension. At least 20% of sub-centimetre nodes ultimately are confirmed to be malignant and around 40% of nodes ‘enlarged’ by CT criteria are benign (18).
Positron Emission Tomography (PET) combined with CT (PET-CT) scanning has revolutionized lung cancer staging and represents the biggest single advance in this field. When available, it should be a routine part of staging in all potentially resectable lung cancers, perhaps with the exception of sub-centimetre screen-detected lesions. PET scanning will often show unexpected uptake in nodal or distant sites. Whilst most of these lesions will be shown to be metastatic deposits, false positive uptake is known to occur, the incidence varying between geographical locations. It is thus important that each unit understands the incidence of false positive uptake in its own population and ensures that no one is denied curative surgery inappropriately. In doubtful cases biopsy of the area of uptake is recommended.
The introduction of endobronchial ultrasound (EBUS) has further revolutionised staging of the mediastinum and for that matter, assessment of all cases of mediastinal adenopathy. It is now possible to diagnose and stage the lung cancer patient in a single outpatient procedure, avoiding ‘diagnostic’ and then ‘staging’ bronchoscopies (19). Surgeons should be driving this process.
In experienced hands EBUS has been shown to be highly sensitive and accurate with a lower complication rate than mediastinoscopy. Yasafuku has demonstrated the equivalence of EBUS transbronchial needle aspiration (TBNA) vs. mediastinoscopy and this would now be the procedure of choice for mediastinal staging (20). Endo-oesophageal ultrasound (EUS) has been used to stage the posterior mediastinum, evaluate the adrenals and even the left lobe of the liver. Whilst a meta-analysis has shown high sensitivity and specificity, the negative predictive value is limited (21). EBUS and EUS have a complementary role to play with reported accuracy of 95%, if available, they play an important role in minimally-invasive mediastinal staging (22).
In many units mediastinoscopy is reserved for the occasional patient where EBUS is negative but clinical suspicion of nodal disease is high, either as a primary staging or after induction therapy, or where mediastinal nodal involvement by sarcoid or lymphoma is suspected but the cores obtained at EBUS are non diagnostic. Surgeons should be performing these themselves and be au fait with on site pathologic assessment or have a close working relationship with physicians skilled in this technique. EBUS has an important role in preoperative determination of N1 disease. Far from irrelevant because it is still ‘surgical’, where resection is considered, N1 positivity may mean pneumonectomy and this has important implications for patient selection. At some centres, patients with N1 disease may undergo preoperative chemotherapy as it is better tolerated than in the adjuvant setting post pneumonectomy and downsizing bulky disease makes for a potentially more satisfactory surgical approach without an increase in morbidity.
The development of video-assisted mediastinal lymphadenectomy (VAMLA) and transcervical extended mediastinal lymphadenectomy (TEMLA) techniques have been described but as yet their role in primary evaluation of the mediastinum remains unclear (23).
VATS staging is occasionally necessary to evaluate a pleural effusion in which repeated aspirates have not confirmed a malignant cause, when nodal status remains unclear, especially in the aorto-pulmonary zone or if pathological confirmation of additional pulmonary nodules is needed to decide appropriate therapy.
In parallel with the assessment of disease extent it is important to assess patient fitness for surgery. Guidelines on pre-operative evaluation of patients outline the efficient way to stage patients to allow decision making on interventions (24).
The ageing population in the developed world has meant that decision making on suitability for surgery is imperative. Risk factors for surgical morbidity and mortality include patient age, sex, American Society of Anaesthesiologists (ASA) score, performance status, surgical priority, comorbidity, induction chemoradiation, forced expiratory volume in 1 second (FEV1), renal dysfunction and body mass index (24). Algorithms on the fitness for surgery have been described by the ACCP, ERS, ESTS and BTS (24-26). Functional assessment includes a walk test and cardiopulmonary exercise testing. Surgeons need to bear in mind though that these tests do have shortcomings and in recognition of this, Lim et al. have proposed greater involvement of the patient in the decision making process (13,27).
In evaluating the trends in surgical resection in England, Riaz et al. noted that the resection rates were increasing despite the patient population becoming older, and that more segmental resections were being performed (28). Increasing age was found to be associated with a decreased likelihood of undergoing pneumonectomy or sleeve resection.
Exploratory thoracotomy rates have also dropped, as have the number of pneumonectomies performed. Five year survival for lobectomy and patients with adenocarcinoma was increased and the overall prognosis over time was found to be improved on multivariate analysis, attributed to earlier diagnosis (28,29).
Surgical technique
Surgical access for lung cancer resection remains topical. Thoracotomy has been the traditional approach for resection of lung cancers. Video-assisted thoracoscopic (VAT) lobectomy generates controversy in the surgical field and has been slow to gain popularity. It is not new, celebrating its 20th anniversary this year. It has evolved and been assisted by improvements in hardware. Advocates argue that the advantages; reduced pain, shortened hospital duration, decreased air leak, pneumonia and atrial arrhythmias favour VATS over traditional thoractomy (30,31). In addition, it has been argued that the increase in inflammatory mediators is less exuberant than with open surgery (32). The counter argument relates to the learning curve safety, patient selection, long term survival and the ability to perform an oncologically complete operation with adequate nodal dissection. The latter has been given increased relevance as the 7th edition of TNM requires a minimum number of lymph nodes to be removed and examined pathologically, to allow a pN category to be assigned and a complete R0 resection to be confirmed (13).
The publication of the phase II CALGB 39802 study established the feasibility of this approach and sought to offer a precise definition (33). It was demonstrated that, with a clear definition of the approach, complications were low and the survival compared favourably to open series. The VATS approach was less expensive, with lower morbidity than cases undergoing thoracotomy. In the absence of large scale randomised controlled trials, systematic reviews and meta-analyses of VATS lobectomy have demonstrated similar benefits (30,31,34). The use of propensity matching, despite some obvious limitations has also been utilised to demonstrate the superior benefits over open thoracotomy (31).
To address the concerns regarding the safety, specifically regarding the management of bleeding, Yamashita et al. published their results with management of intra-operative vessel injury. In a review of 557 patients, there were 26 (4.7%) vascular injuries, 17 of which involved pulmonary arterial branches. Fifty percent of these required conversion to thoracotomy and another 23% required mini-thoracotomy. They also noted no differences in hospital stay and overall morbidity but an increase in surgical time and blood loss (35). This led to the conclusion that safety concerns were not significant enough to preclude the VATS approach.
Hanna et al. compared cancer specific and overall survival in a propensity matched cohort of 190 VATS patient with open lobectomy (36). No statistically significant difference in cancer specific (76.7% vs. 82.9%, P=0.170) or overall survival (64% vs. 73%, P=0.170) was detected. Operative mortality and morbidity were similar in the 2 groups.
In a meta-analysis comparing the long term survival in patients undergoing VATS (n=2,106) and open surgery (n=2,661), Taioli et al. reviewed 20 observational studies. Long term survival was found to be increased in the VATS group with a 5% meta difference (95% CI, 3-6%) (37). Further evidence for at least an equivalent disease-free and overall survival was also provided by Kuritzky et al. (38).
Despite this, the uptake of this approach has been relatively slow. In a review of the STS General Thoracic Database, Paul et al. noted that in 2007, only 30% of all lobectomies were performed thoracoscopically (39).
Subsequent review of Nationwide Inpatient Sample Database by the same author encompassing 2007-2008, demonstrated that only 15% of lobectomies were thoracoscopic (40). Interestingly, the majority of these procedures (67%) were performed in teaching hospitals. Clear consensus on, and compliance with the definition of VATS lobectomy has hampered progress. Furthermore it is never clear what the comparator is for the open approach. Traditional posterolateral thoracotomy for lung cancer resection is clearly obsolete but this is often the “gold standard” against which VATS lobectomy is compared. The randomised trials do not address the observation that an experienced high volume surgeon, using a small 6-8 cm incision with limited rib spreading and standard techniques can achieve outcomes with open surgery equivalent or better than those published for VATS with less cost. Enthusiasm for ‘minimally invasive’ procedures needs to be tempered with tight cost evaluation and data that applies to the wider surgical community rather than specialist academic centres.
In an editorial commenting on VATS lobectomy, Wood noted that this procedure is still generally performed in high volume and academic centres. It is postulated that the improved outcomes noted in most studies relates to the surgeon rather than to the actual procedure (41). A similar point was raised by Farjah et al. noted that there was a higher hazard of death after VATS with low-volume surgeons (42). This is concerning and in our opinion likely to be under reported.
Many surgeons performing lung cancer surgery can do a safe operation with acceptable outcomes. The translation to the VATS approach however is not straightforward. Surgeons should look at their own results and outcomes. If they are equivalent (or better) to those published for VATS, they should not be under pressure to change technique. The vocal proponents of the VATS approach generally have set the baseline for the outcomes studies.
VATS lobectomy has clearly been demonstrated to be safe and oncologically effective, and more radical procedures are also being performed via this route. It is expected that uptake will increase with greater exposure during training of junior surgeons making it the standard of care in the future (43).
The extension of the multi-port approach is the single port technique pioneered initially by Rocco and now by Gonzales-Rivas (44,45).
The lobectomy performed through the uniport incision follows standard procedure with individual ligation of vascular structures and bronchus with mediastinal nodal dissection. Visualisation is entirely via the videoscope. The technique has been well described (45). Advantages include vision directed to the target tissue, similar to open surgery and reduction of post-operative pain. Uniportal VATS limited resections (wedge) can even be performed under locoregional anaesthesia in awake patients (46). The impending increase in referrals from the advent of screening programmes makes this approach important for the future. Clearly there is a learning curve and it has been suggested that surgeons already performing VATS lobectomy via the anterior approach may adapt to this technique earlier. Once again, surgeons must evaluate their current practice and make an assessment whether such a technique would be useful to them.
Robotic surgery is an extension of the VATS minimally invasive approach. Proponents argue that there is improved operative field visualisation and better wrist-like motion of the instruments with tremor filtration (47,48). Disadvantages include the added cost associated with the robotic technology. Proponents of the VATS approach also believe that it does not add more to an already well established surgical approach. Evaluating the robotic approach in a systematic review, Cao et al. found that the robotic procedure was safe and effective in specialized centres. Long term efficacy data was limited however and warranted further study (49). Procedures are generally performed via 2-3 ports and a utility incision with no rib spreading, generally following the CALGB VATS technique. This approach still allows lymph node dissection. Studies have demonstrated similar operative times after the initial learning curve, reduced hospitalization and time to normal activity with some evidence for reduced post-operative pain. Equivalent oncological results to open thoracotomy have also been reported (50). The rate of conversion to open thoracotomy has been reported at 7-8% (47-52) In the largest reported series on robotic lobectomy, Park et al. reported an 80% overall 5-year survival with equivalent stage specific survival data compared to VATS approach. Whilst this was in a retrospective study that did not directly compare the 2 approaches, it still demonstrates the oncological efficacy of robotic lobectomy (53).
Whilst technically feasible, widespread uptake in an era of cost containment is highly unlikely. It is a nice marketing tool where competition for patients is high. The same concerns regarding learning curve, training etc. for VATS exist here but are even more pertinent (54).
How much lung is enough? The role of sub-lobar resection
Sub-lobar resections consist of anatomical segmental resection and wedge resections that are non-anatomical. Wedge resections generally have a poorer outcome compared to anatomical resection. Nakamura et al. reported a 55.4% 5-year survival after wedge excision, lower compared to lobectomy (82.1%) and segmental resection (87.2%) (55). It is with this in mind that the rest of this discussion will focus mainly on anatomical segmentectomy.
Segmental resections for early stage lung cancer have traditionally been reserved for patients with limited functional reserve, medical co-morbidity and for older patients. This is largely in view of the only randomized trial available comparing lobectomy to sub-lobar resection by Ginsberg et al. for the Lung Cancer Study Group (56). They demonstrated an inferior 5-year survival with limited resection as well as a threefold increase in local recurrence rates for tumours smaller than 3 cm confirmed to be N0 at thoracotomy. The locoregional recurrence rate per person/year was 0.044 for segmental resection and 0.086 for wedge resections. This study was limited by the low number included in the study and the unavailability of PET at this time.
Wolf et al. reported their experience with segmental resections compared to open lobectomy. They found a trend to increased local recurrence with shorter overall and recurrence free survival in segmental resections (57). This survival difference should be taken in context though; there were a larger number of older patients with poor lung function in the segmental group.
In a meta-analysis comparing survival to lobectomy for stage I disease, Nakamura et al. demonstrated better survival, albeit not statistically significant, following lobectomy. There was however considerable heterogeneity at time points 3 and 5 years after resection (58).
Interpretation of the data is difficult as there are differences in the application of segmental resection, as well as the extent of mediastinal nodal dissection at the time of resection. Tumour histology also plays a role in the outcomes with slow growing adenocarcinoma demonstrating better results.
In the absence of randomised controlled trials, Tsutani et al. published a propensity matched analysis limited to patients with stage IA lung adenocarcinoma (59). They excluded wedge resections and demonstrated no difference in survival and recurrence free survival in all cohorts before and after propensity matching. Of note was the fact that they included segmental resections for TIb tumours based on the standardized uptake value (SUVmax) and high resolution computerised tomography (HRCT) findings. Solid tumour size on HRCT and lower SUVmax were independent prognostic factors and tended to predict less invasive tumours that were managed by segmentectomy.
Reviewing the Surveillance, Epidemiology and End Result (SEER) database, Kates et al. examined the survival outcomes following lobectomy and segmentectomy for stage I tumours up to 1 cm. They noted equivalent survival and commented that segmentectomy may be preferable given the lower rate of complications. No survival difference could be demonstrated before and after propensity matching (60). Yang and D’Amico reviewed the results of thoracoscopic segmentectomy for lung cancer. The trends in the literature suggest that this approach, specifically for early stage tumours and the low-grade, ground glass opacity (GCO) cases, which if < than 3 cm are now classified as adenocarcinoma in situ (AIS), is safe and feasible (61). Zhong et al. demonstrated similar local recurrence and equivalent 5-year survival comparing thoracoscopic segmentectomy and lobectomy (62). The role of segmental resection is clearly being defined. Gorenstein et al. reviewed surgery for early stage cancer suggesting the following indications for sublobar resection (Table 1) (63). There are currently 2 randomised controlled trials that may clarify the role of limited resection, the CALGB 140503 (segmentectomy and wedge resection) and JCOG0802/WJOG4607L (segmentectomy). In these trials, selection for limited resection includes tumours 2 cm or less in size, peripheral tumours close to the outer third of the lung and good functional status. These results are awaited (64,65).

Full Table
Lung preservation is also behind the push for sleeve resections, either bronchial, pulmonary arterial or the double sleeve resection. These are indicated for tumour involving either the origin of a lobar bronchus or lobar branch of the pulmonary artery that does not infiltrate as far as to require pneumonectomy. It allows patients who would not tolerate a pneumonectomy to undergo curative resection. D’Andrilli found that the oncological efficacy of sleeve resection is well established in stage I and II disease with some benefit in stage III over pneumonectomy. Quality of life, prognosis and morbidity were better in patients undergoing sleeve resection compared to pneumonectomy (66). Outcomes following resection and reconstruction of the pulmonary artery have been shown to be similar to standard lobectomy in selected patients (67).
Nodal dissection
There is ongoing controversy on the role of mediastinal node dissection during the resection. Nodal dissection allows accurate staging for prognostic purposes, thereby determining the need for adjuvant therapy and is necessary to ensure a complete R0 resection as defined by Rami-Porta et al. (68). There is good evidence of improved “stage specific” survival as the number of nodes removed increase. It also removes microscopic nodal disease that may result in local recurrence. The extent of this nodal dissection has long been the subject of discussion.
Wu et al. reported improved overall survival in patients undergoing systematic nodal dissection (SND) which is the only internationally standardized technique for intrathoracic nodal evaluation (69-71). The ACOSOG Z0030 trial reported no difference in survival between patients thought to have no nodal disease or non-hilar N1 disease randomised to nodal sampling or more extensive nodal dissection (72). Of note is the fact that the ACOSOG Z0030 utilised intra-operative frozen section analysis to ensure negative nodal status before randomisation. This is the practice of one of the authors (PG) as well.
In a retrospective review, Cerfolio et al. documented a higher rate of N2 pick-up with mediastinal nodal dissection with no impact on survival (73). This was in normal day to day surgical practice with no intra-operative frozen section.
Arguments against the routine mediastinal nodal dissection include the possibility of increased operative time or post-operative morbidity, a finding not supported by the ACOSOG Z0030. The 7th edition of TNM recommends that assessment of regional lymph node involvement be performed by the removal and subsequent pathological examination of a minimum of 6 nodes/stations, 3 from the mediastinum, including the subcarinal node (#7), and 3 from N1 zones.
Locally ablative therapy
Sub-lobar resection faces challenges from less invasive medical procedures. These include thermal ablation, either radio-frequency (RFA) or microwave, and stereotactic body radiotherapy (SBRT or stereotactic ablative radiotherapy—SABR) which has demonstrated excellent primary tumour control which some say approaches that of lobectomy (74,75).
RFA is currently utilised for medically inoperable patients with early stage tumours, either stage I or II. It has also been used to manage patients with pulmonary metastases if <5 cm. Reports on the long term benefits are limited though. In an editorial by Fernando, questions on the role of RFA over SBRT are raised and highlight the possible deficiencies facing RFA (76).
Stereotactic body radiotherapy is mainly in patients deemed high risk for surgery. Senan et al. have demonstrated the efficacy of SABR for early NSCLC in medically inoperable patients (77). The data is based on progression-free survival which is a concern given the acknowledged difficulties in assessing progression after such treatment. In addition local progression may be under-estimated if patients are not returned to the specialized centre for follow up. The diagnosis of malignancy was only confirmed in 31% of cases reported by Lagerwaard et al., a further area of concern (78). In a retrospective review of the SEER database, Fernandez et al. compared definitive radiation with sublobar resection in stage IA disease. The 3-year survival favoured sublobar resection in this cohort of high risk patients (79).
A phase III trial is currently underway comparing the sublobar resection with SBRT in high risk stage I disease (80). Patients will be randomised to either treatment arm but interestingly, no routine pre-operative mediastinal nodal staging will be performed which will result in some of the surgical arm being upstaged by nodal dissection. Despite this confounding factor the trial should help define the role of SBRT.
Lung cancer screening
The impact of Low-Dose CT (LDCT) screening for lung cancer will result in large numbers of patients being referred for the evaluation of nodules, many of which will not be malignant. Such evaluation requires a dedicated multidisciplinary approach if invasive investigations and resection for benign disease is to be kept at an acceptably low level. Such screening programmes will inevitably lead to an increased volume of patients with small lung cancers (1-2 cm) being presented for possible surgical resection.
The benefits of screening with low-dose CT scans are largely based on the results of The National Lung Screening Trial (NLST) published in 2011 (81). Comparing LDCT to chest radiographs, there was a 20.3% reduction in lung cancer related mortality and a 7% overall reduction in mortality. A caveat though was the false positivity rate of 95% in the NLST screening trial at prevalence screen. Surgical involvement in screening programmes is critical as it is anticipated that the number of referrals will increase.
Guidelines on intervention are currently available from the IALSC (82). Key recommendations include the use of a multidisciplinary team approach with surgery being performed in centres with minimally invasive programmes. Surgical resection, once diagnosis is confirmed, should also be anatomical by lobectomy with SND. Segmentectomy, and even wedge excision might be appropriate for (I) pure ground-glass opacities which if <3 cm with no invasive element and pure lepidic growth are now classified as “adenocarcinoma in-situ” and as such have almost 100% cure rate, and (II) screen-detected part-solid lesions <2 cm in the outer one third of the lung in whom frozen section has confirmed N0 disease and in which resection margins are checked by cytology of frozen section. The results of the JCOG and CALGB studies on segmentectomy may require us to re-evaluate these recommendations, especially as one becomes more concerned about second primaries in patients with high probability of cure from their first cancer.
Indeterminate lesions will require tissue for diagnosis, with CT-guided biopsy being encouraged. The decision to intervene will depend on the probability of lung cancer. It has been shown that lesions >20 mm have an 80% probability of being malignant. The risk of malignancy is reduced with numerous nodules (>6). Part solid (63%), non-solid (18%) and solid (7%) lesions all have varying degrees of malignancy associated with them (83).
We must await the results of ongoing trails, especially in Europe with the Dutch-Belgium randomised NELSON trial and the Danish Lung Cancer Screening Trial to see if the NLST translates across geographical regions and is cost-effective in varied health care systems (84,85).
Locally advanced disease: the role of surgery in a Multi-Modality setting
There remains significant controversy as to the role of surgery in locally advanced disease but for most surgeons resection performed as part of a multimodal therapy remains the cornerstone for any chance of cure for this group.
Stage III NSCLC represents a heterogeneous group and this is recognized by the recent American College of Chest Physicians (ACCP) clinical practice guidelines (86). Most surgeons would feel that isolated single N2 station nodal metastasis, assessed as ‘resectable’ should be considered for a treatment plan to include chemotherapy and surgical resection with or without thoracic radiotherapy which, whilst more contentious, is making a comeback. The order of the tri-modality therapy is variable. Neoadjuvant chemotherapy has been shown to improve survival compared to surgery alone. Two landmark studies have compared the results of surgery alone in N2 disease versus the impact of neoadjuvant chemotherapy and surgery. Roth et al. showed a 5-year survival of 15% with surgery alone compared to 36% after pre-operative chemotherapy (87), and Rosell et al. obtained a significant overall survival advantage in the combined group (3-year survival of 15%) over surgery alone (3-year survival 0%) (88). Whilst the advantage to neoadjuvant chemotherapy in both arms is similar, an approximately 20% improvement in overall survival, the marked difference in the results of surgery alone (0% versus 15%) suggests the populations of N2 disease entered in to each trial differed significantly.
Recently, Ripley and Rusch have published an authoritative review of the role of induction therapy. After an extensive review of the current best available evidence they conclude that multimodality therapy should be standard of care for stage IIIA (N2) NSCLC, resection being offered to patients suitable for complete resection (89).
Randomised controlled trials of multimodality therapy in pre-operatively determined N2 disease, comparing regimens which included surgery with those using only chemotherapy and radiotherapy have shown the results to be similar. Arguments against the role of surgery in N2 disease cite Van Meerbeck et al., however, looking at the surgical group in this series, it was suggested that surgery less than pneumonectomy may provide a survival advantage (90).
Many surgical oncologists would agree however that the wide variety of findings mandates individualized assessment and treatment planning by a team experienced in lung cancer surgery. Similarly therefore, a smaller peripheral primary tumour and a single paratracheal or subcarinal metatstasis that would require a very large radiation field for radical treatment may be better treated with a multimodality approach including surgery. Finally, bulky central tumours with uncertain resectability (Likely T4) may be better treated with initial chemotherapy/chemoradiotherapy followed by surgical exploration once some cytoreduction has been achieved. However, there is concensus that bulky multi-station disease is better treated with definitive, concurrent chemoradiation. These special treatment issues are also well described in the ACCP guidelines (91).
There have been a significant number of clinical trials evaluating preoperative chemoradiation followed by surgery for locally advanced NSCLC. The most influential of these, including the SWOG 8805, German and Massachusetts General have shown increased resectablity rates, increased but acceptable perioperative morbidity and mortality with survival benefit (92-94). In the Prince Charles Hospital it is our preference to use induction chemotherapy alone and reserve surgery post chemoradiation as a salvage option only.
Oncologists in general, conclude from the EORTC 08941, that surgery does not improve survival in patients with N2 disease and therefore should not be used (95). Referral of patients with low volume N2 disease has been limited, which, in our opinion means denying these patients access to better treatment. Better local control occurred in the surgical arm and patients having an R0 resection had improved survival.
Further, the Intergroup trial 0139 showed no overall difference in survival in the surgical arm (96). There was a high mortality rate in the trial after pneumonectomy however and a clear survival advantage was present for patients having lobectomy after induction therapy, findings supporting the ‘unplanned’ analysis of the surgical group in the EORTC 08941. The differences in 5-year survival between the intergroup study and the EORTC are greater than can be explained by the difference between sequential and concurrent chemoradiation suggesting that the study populations were somewhat different. Weder et al. demonstrated that pneumonectomy can be performed with very acceptable morbidity and mortality after induction therapy (97). This further emphasizes the importance of such cases being dealt with by experienced multidisciplinary teams.
The T4 descriptor in the staging system has generally signified ‘irresectable’ disease. Whilst there are undoubtedly cases in which T4 cases, especially when associated with N0 of N1 disease (now stage IIIA in the 7th edition) can be resected and benefit from surgical treatment, it is difficult to support this in the literature since the case series are small and include an unspecified number of cases that were not characterized as T4 before surgery. In many cases in it difficult to be sure without exploration whether this is the case. Each of these patients needs to be assessed individually. Treatment options are between radical intent chemoradiation or surgery. Some centres may opt for surgical exploration and a ‘trial dissection’. In our unit, in some cases, chemotherapy is given before exploration. Responders will undergo exploration with the aim of complete resection. In some units there is a move toward preoperative chemoradiation (98) with which there is little doubt that perioperative morbidity and mortality is higher, as is the pathologic complete response rate. Patient assessment should be in experienced units. Surgery for Pancoast tumour is well established as part of multimodality therapy. Induction chemoradiation followed by complete surgical resection is the current standard of care.
Improvements in staging technologies have undoubtedly resulted in more patients being identified with unexpected and limited metastatic disease. The standard of care for stage IVB cases is definitive chemotherapy. Adjuvant surgery may be considered in occasional and highly selected patients with oligometastatic disease such as solitary brain and adrenal metastases. There are small series suggesting improved disease-free and overall survival in these circumstances (99,100). This radical approach would only be considered in the uncommon scenario of a resectable, node negative primary with an isolated metastatic deposit. Chemotherapy is an integral component in such cases and in our department would usually be administered after resection of the metastasis and before definitive pulmonary resection.
Adjuvant chemotherapy
Recurrence after complete resection for lung cancer is most commonly at distant sites.
There have been 4 positive adjuvant trials from 1994-2001 demonstrating a survival benefit with adjuvant chemotherapy (101-103). The greatest benefit was shown in the National Cancer Institute of Canada JBR.10, however, subset analysis showed no benefit for stage IB patients (104). These results were confirmed in the recent update of the trial results (105). The survival benefit reported in this trial was 15% at 5 years.
The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis demonstrated a trend to benefit in stage IB and clear benefit in stage II N1 cases and IIIA mostly N2 cases disease (106).
Adjuvant chemotherapy has become the standard for resected stage II and IIIA disease, with level 1 evidence for cisplatin based chemotherapy in these patients. It has been suggested (level 2B) that high risk stage IB disease including; poorly differentiated carcinoma, vascular invasion, wedge resection and visceral pleural involvement, should also be offered treatment (101,102). In considering these results, it is worth remembering that in all these positive trials the 6th edition of the staging system was used. The benefit for Stage II was therefore in cases with N1 disease and in the stage IIIA cases with N2 disease. The CALGB 9633 trial for 6th edition stage IB was negative and only an unplanned post hoc analysis showed benefit for N0 cases 4 cm or larger. This finding in large N0 cases was not supported when Shepherd et al pooled the data from the JBR 10 and CALGB 9633 trials (103). It appears clear that adjuvant chemotherapy offers benefit in node positive cases, the role in bulky but node negative cases is uncertain.
It is imperative that surgeons are familiar with this data as they are best placed to assess the suitability for adjuvant therapy. Whilst there is no conclusive data to suggest that neoadjuvant chemotherapy would be better than adjuvant chemotherapy there are reasons to think that it may be more effective (107). There is improved drug delivery to the tumour, particularly lymph nodes, better prospect of receiving the full dose of the planned regimen, earlier treatment of micrometastatic disease, and the possibility of improved resectability. For patients with bulky N1 disease requiring pneumonectomy in our institution neoadjuvant chemotherapy followed by resection is the preferred approach.
The CALGB 150803 trial is currently underway attempting to identify a subset of stage I patients that benefit from adjuvant chemotherapy using a 64-gene signature.
Small cell lung cancer
Small cell lung cancer represents 13% of newly diagnosed cases of lung cancer worldwide (108). It is common in heavy smokers, either current or previously, and is associated with early locoregional and distant spread. Treatment modalities are commonly limited to a combination of thoracic irradiation and multi-agent chemotherapy with surgery having a limited role.
Surgery was initially advocated based on the results of the Veterans Administration Surgical Oncology Group in 1982 with a 60% 5-year survival for T1N0 lesions with surgery and chemotherapy (109).
The Lung Cancer Study Group prospective trial of induction chemotherapy followed by either surgery or radiation demonstrated no survival benefit in either treatment arm, but excluded patients with stage I disease (110). It is one of the few randomised trials looking at the role of surgery.
Surgery is recommended for biopsy proven T1N0M0 disease, with adjuvant chemotherapy and prophylactic cranial irradiation (110). It may also be offered after neoadjuvant therapy (111,112). Surgery may also be offered as a salvage option to patients with relapse after remission or non-responders, Shepherd et al. reported a retrospective review with a 23% 5-year survival (113). There have also been selected reports of surgery for extensive disease with staging and selection being critical.
Conclusions
Surgical therapy for lung cancer has advanced since the first pneumonectomy by Evarts Graham (114). Advances in pre-operative, operative and post-operative care have revolutionized management and improved outcomes.
Multi-modality therapy, an expanding role for adjuvant therapy after complete resection and medical alternatives to surgery require that surgeons take an active role in the multidisciplinary discussions. They need to be fully conversant with the available literature and capable of strongly presenting the benefits of surgical options. The expanding use of LDCT screening will involve surgeons in the evaluation and treatment of smaller cancers which force us to re-evaluate our investigative algorithms and surgical options. Sub-lobar resections, minimally invasive strategies with earlier intervention for stage IA disease together with extending the role of surgery in advanced stages point the way forward for the thoracic surgical community.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 10 (Internet). Lyon, France: International Agency for Research on Cancer; 2010. Available online: , accessed on 7/04/2013.
- Australian Institute of Health and Welfare & Australasian Association of Cancer Registries 2012. Cancer in Australia: an overview, 2012. Cancer series no. 74. Cat. no. CAN 70. Canberra: AIHW.
- Australian Institute of Health and Welfare 2012. Cancer survival and prevalence in Australia: period estimates from 1982 to 2010. Cancer series no. 69. Cat. No. CAN 65. Canberra: AIHW.
- Drilon A, Rekhtman N, Ladanyi M, et al. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol 2012;13:e418-26. [PubMed]
- Rekhtman N, Brandt SM, Sigel CS, et al. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol 2011;6:451-8. [PubMed]
- Koudelakova V, Kneblova M, Trojanec R, et al. Non-small cell lung cancer - genetic predictors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2013;157:125-36. [PubMed]
- Bunn PA Jr. Worldwide overview of the current status of lung cancer diagnosis and treatment. Arch Pathol Lab Med 2012;136:1478-81. [PubMed]
- Ou SH, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 2012;17:1351-75. [PubMed]
- Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med 2011;32:22-31. [PubMed]
- Mazzone P. Preoperative evaluation of the lung resection candidate. Cleve Clin J Med 2012;79 Electronic Suppl 1:eS17-22.
- Gorenstein LA, Sonett JR. The surgical management of stage I and stage II lung cancer. Surg Oncol Clin N Am 2011;20:701-20. [PubMed]
- Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax 2006;61:597-603. [PubMed]
- Gospodarowicz MK, O’Sullivan B, Koh ES. Prognostic factors: Principles and applications. In: Peter Goldstraw. eds. IASLC Manual - Staging Manual in Thoracic Oncology. Orange Park, FL, USA: Editorial Rx Press, 2009:111-28.
- Daniels AC. A method of biopsy useful in diagnosing certain intrathoracic diseases. Dis Chest 1949;16:360-7. [PubMed]
- Carlens E. Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest 1959;36:343-52. [PubMed]
- McNeill TM, Chamberlain JM. Diagnostic anterior mediastinotomy. Ann Thorac Surg 1966;2:532-9. [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6. [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S.
- Fielding D, Windsor M. Endobronchial ultrasound convex-probe transbronchial needle aspiration as the first diagnostic test in patients with pulmonary masses and associated hilar or mediastinal nodes. Intern Med J 2009;39:435-40. [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1.
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [PubMed]
- Herth FJ, Lunn W, Eberhardt R, et al. Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. Am J Respir Crit Care Med 2005;171:1164-7. [PubMed]
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [PubMed]
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [PubMed]
- Lim E, Beckles M, Warburton C, et al. Cardiopulmonary exercise testing for the selection of patients undergoing surgery for lung cancer: friend or foe? Thorax 2010;65:847-9. [PubMed]
- Riaz SP, Linklater KM, Page R, et al. Recent trends in resection rates among non-small cell lung cancer patients in England. Thorax 2012;67:811-4. [PubMed]
- Riquet M, Berna P, Fabre E, et al. Evolving characteristics of lung cancer: a surgical appraisal. Eur J Cardiothorac Surg 2012;41:1019-24. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [PubMed]
- Yamashita S, Tokuishi K, Moroga T, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non-small cell lung cancer. Ann Thorac Surg 2013;95:994-9. [PubMed]
- Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013;43:1121-5. [PubMed]
- Taioli E, Lee DS, Lesser M, et al. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg 2013;44:591-7. [PubMed]
- Kuritzky AM, Ryder BA, Ng T. Long-term survival outcomes of Video-assisted Thoracic Surgery (VATS) lobectomy after transitioning from open lobectomy. Ann Surg Oncol 2013;20:2734-40. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [PubMed]
- Wood DE. What is most important in improving outcomes after pulmonary lobectomy: the surgeon or the approach? Eur J Cardiothorac Surg 2013;43:817-9. [PubMed]
- Farjah F, Flum DR, Varghese TK Jr, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg 2009;87:995-1004. [PubMed]
- Puri V, Meyers BF. Video-assisted thoracoscopic surgery lobectomy for lung cancer. Surg Oncol Clin N Am 2013;22:27-38. [PubMed]
- Rocco G. One-port (uniportal) video-assisted thoracic surgical resections-- a clear advance. J Thorac Cardiovasc Surg 2012;144:S27-31. [PubMed]
- Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach. ScientificWorldJournal 2012;2012:780842.
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg 2010;89:1625-7. [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [PubMed]
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6. [PubMed]
- Swanson SJ. Robotic pulmonary lobectomy--the future and probably should remain so. J Thorac Cardiovasc Surg 2010;140:954. [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2011;59:137-41. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5.
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer 2005;92:1033-7. [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [PubMed]
- Yang CF, D’Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [PubMed]
- Gorenstein LA, Sonett JR. The surgical management of stage I and stage II lung cancer. Surg Oncol Clin N Am 2011;20:701-20. [PubMed]
- CALGB 140503. A phase III randomised trial of lobectomy versus sublobar resection for small (≤ 2 cm) peripheral non-small cell lung cancer. Available online: , accessed April 22,2013.
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [PubMed]
- D’Andrilli A, Venuta F, Menna C, et al. Extensive resections: pancoast tumors, chest wall resections, en bloc vascular resections. Surg Oncol Clin N Am 2011;20:733-56. [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001. [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [PubMed]
- Yl Wu. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [PubMed]
- Graham AN, Chan KJ, Pastorino U, et al. Systematic nodal dissection in the intrathoracic staging of patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 1999;117:246-51. [PubMed]
- Nicholson AG, Graham AN, Pezzella F, et al. Does the use of immunohistochemistry to identify micrometastases provide useful information in the staging of node-negative non-small cell lung carcinomas? Lung Cancer 1997;18:231-40. [PubMed]
- Darling GE, Allen MS, Decker P, et al. Randomised trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in patients with N0 or N1 (less than hilar) non-small cell carcinoma:results of the ACOSOG Z0030 trial. AATS Annual Meeting. Toronto: Canada, 2010.
- Cerfolio RJ, Bryant AS, Minnich DJ. Complete thoracic mediastinal lymphadenectomy leads to a higher rate of pathologically proven N2 disease in patients with non-small cell lung cancer. Ann Thorac Surg 2012;94:902-6. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [PubMed]
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427-31. [PubMed]
- Fernando HC. The present and future of thermal ablation for lung cancer. Eur J Cardiothorac Surg 2013;43:687. [PubMed]
- Senan S, Palma DA, Lagerwaard FJ. Stereotactic ablative radiotherapy for stage I NSCLC: Recent advances and controversies. J Thorac Dis 2011;3:189-96. [PubMed]
- Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685-92. [PubMed]
- Fernandez FG, Crabtree TD, Liu J, et al. Sublobar resection versus definitive radiation in patients with stage IA non-small cell lung cancer. Ann Thorac Surg 2012;94:354-60. [PubMed]
- Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;144:S35-8. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Field JK, Smith RA, Aberle DR, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol 2012;7:10-9. [PubMed]
- Stiles BM, Altorki NK. Screening for lung cancer: challenges for the thoracic surgeon. Surg Oncol Clin N Am 2011;20:619-35. [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [PubMed]
- Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608-14. [PubMed]
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-40S.
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [PubMed]
- Ripley RT, Rusch VW. Role of induction therapy: surgical resection of non-small cell lung cancer after induction therapy. Thorac Surg Clin 2013;23:273-85. [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [PubMed]
- Choi NC, Carey RW, Daly W, et al. Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J Clin Oncol 1997;15:712-22. [PubMed]
- Eberhardt WE, Albain KS, Pass H, et al. Induction treatment before surgery for non-small cell lung cancer. Lung Cancer 2003;42:S9-14. [PubMed]
- Splinter TA, van Schil PE, Kramer GW, et al. Randomized trial of surgery versus radiotherapy in patients with stage IIIA (N2) non small-cell lung cancer after a response to induction chemotherapy. EORTC 08941. Clin Lung Cancer 2000;2:69-72. [PubMed]
- Uy KL, Darling G, Xu W, et al. Improved results of induction chemoradiation before surgical intervention for selected patients with stage IIIA-N2 non-small cell lung cancer. J Thorac Cardiovasc Surg 2007;134:188-93. [PubMed]
- Weder W, Collaud S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010;139:1424-30. [PubMed]
- D’Angelillo RM, Trodella L, Ciresa M, et al. Multimodality treatment of stage III non-small cell lung cancer: analysis of a phase II trial using preoperative cisplatin and gemcitabine with concurrent radiotherapy. J Thorac Oncol 2009;4:1517-23. [PubMed]
- Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg 1996;84:748-54. [PubMed]
- Louie AV, Rodrigues G, Yaremko B, et al. Management and prognosis in synchronous solitary resected brain metastasis from non-small-cell lung cancer. Clin Lung Cancer 2009;10:174-9. [PubMed]
- Coleman MH, Bueno R. Role of adjuvant chemotherapy in NSCLC (stages I to III). Surg Oncol Clin N Am 2011;20:757-67. [PubMed]
- Reungwetwattana T, Eadens MJ, Molina JR. Chemotherapy for non-small-cell lung carcinoma: from a blanket approach to individual therapy. Semin Respir Crit Care Med 2011;32:78-93. [PubMed]
- Cuffe S, Bourredjem A, Graziano S, et al. A pooled exploratory analysis of the effect of tumor size and KRAS mutations on survival benefit from adjuvant platinum-based chemotherapy in node-negative non-small cell lung cancer. J Thorac Oncol 2012;7:963-72. [PubMed]
- Pepe C, Hasan B, Winton TL, Seymour L, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol 2007;25:1553-61. [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [PubMed]
- Shields TW, Higgins GA Jr, Matthews MJ, et al. Surgical resection in the management of small cell carcinoma of the lung. J Thorac Cardiovasc Surg 1982;84:481-8. [PubMed]
- Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320S-323S. [PubMed]
- Goldstein SD, Yang SC. Role of surgery in small cell lung cancer. Surg Oncol Clin N Am 2011;20:769-77. [PubMed]
- Badzio A, Kurowski K, Karnicka-Mlodkowska H, et al. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg 2004;26:183-8. [PubMed]
- Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. [PubMed]
- Shepherd FA, Ginsberg R, Patterson GA, et al. Is there ever a role for salvage operations in limited small-cell lung cancer? J Thorac Cardiovasc Surg 1991;101:196-200. [PubMed]
- Graham EA. The first total pneumonectomy. Tex Cancer Bull 1949;2:2-4. [PubMed]