Impact of pregabalin on early phase post-thoracotomy pain compared with epidural analgesia
Introduction
Post-thoracotomy pain is found in 20% to 80% patients who have undergone thoracotomy using a chest retractor through the intercostal space (1-9). Epidural analgesia is known to be the gold standard for controlling pain during the perioperative period (10-12); however, its effectiveness has not been satisfactory after thoracotomy (7,13,14). Moreover, epidural analgesia increases the risk of spinal injury and bleeding, making it unsuitable for patients who are receiving antithrombotic therapy (15-17). The use of epidural analgesia for long periods is not recommended due to spinal infection risk (18). Therefore, the development of a safe pain management alternative to epidural analgesia which can be administered for extended periods of time is desirable. Pregabalin is a therapeutic drug for neuropathic pain which interacts with presynapses and would be anticipated to show an effect similar to epidural analgesia (19-23). We have reported that pregabalin is effective against post-thoracotomy pain (24-26). Therefore, we hypothesized that pregabalin would have an analgesic effect, which may be used as an alternative to epidural analgesia in thoracotomy. In this study, a prospective study to compare the effects of pregabalin with epidural analgesia on acute post-thoracotomy pain was conducted, as well as an investigation of the safety and efficacy of pregabalin.
Methods
This prospective and randomized controlled study was approved by the Institutional Ethics Review Board (Teikyo University Review Board 13-122), and the study was registered on the University Hospital Medical Information Network (UMIN000013189). All participants provided written informed consent prior to the study.
This study was performed based on clinical research previously conducted using pregabalin (26). Details including measurement methods are referenced in a previous study (26). This study was designed to include adult patients scheduled to undergo thoracotomy. The inclusion criteria of this study were: (I) both sexes; (II) aged 20 to 80 years old; (III) Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0 or 1; (IV) normal renal function with creatinine clearance >30 mL/min. And the exclusion criteria were: (I) previous administration of antidepressants, anticonvulsants, or opioids before surgery; (II) serious medical conditions (uncontrolled heart, lung, liver or kidney disease or diabetes); (III) a history of angioedema; (IV) pregnant or nursing; (V) problematic in receiving epidural anesthesia (spinal deformity, or previous anticoagulation therapy).
Patients were randomly assigned to a treatment group (pregabalin group) or a control group (epidural analgesia group) using a computer-generated randomization sequence (Nippon RAD Inc., Tokyo, Japan). Patients in the pregabalin group received 75 mg pregabalin orally twice daily starting from the day of surgery. For patients with a creatinine clearance <60 mL/min, 75 mg of pregabalin was orally administered once in the evening. Patients in the epidural analgesia group received 5 mL of 0.3% ropivacaine hydrochloride at the start of procedures as an initial bolus, followed by hourly bolus of the same dose during the surgery. For the postoperative analgesia, 0.2% ropivacaine hydrochloride and 1 µg/mL fentanyl through a thoracic epidural catheter by an epidural patient-controlled analgesia (PCA) pomp was commenced since the end of operations and continued for 48 hours. The PCA pomp was programmed to administer 5 mL/h and for PCA bolus, 3 mL with a lockout of 30 min. A catheter was introduced 6 to 8 cm into the epidural space between T7‒T9 before induction of anesthesia. The loss of resistance technique was used to identify the epidural space, and response to cold stimulation was used to evaluate blockade. In both groups, patients received oral administration of 200 mg of celecoxib twice daily starting from the day of surgery. Oral administration was continued for 5 days after surgery. A primary doctor determined whether or not patients required an additional analgesic drug, where the analgesic drug was discontinued for patients showing numerical rating scale (NRS) of less than 3.
An anesthesiologist was fully responsible for administering anesthetics during surgery, and the surgery was conducted under general anesthesia. Local anesthesia by an intercostal nerve block was not administered during surgery. We commonly perform a muscle-sparing thoracotomy at the 4th or 5th intercostal space using a chest retractor where a skin incision is made from the lateral to the anterior axillary line with a length between 8 and 10 cm to preserve the latissimus dorsi and the serratus anterior muscles.
The primary outcome was the severity of post-thoracotomy pain, while secondary outcomes were severity of sleep interference, use of additional analgesic drugs, development of adverse effects as well as time required in the operating room, anesthetic induction time, operation time, and recovery time.
Patients were asked to rate their pain (NRS) for the previous 24 hours and their sleep interference rate (SIR) during the previous night, and circled numbers which best described on an 11-point scale, where 0 indicated no pain or sleep interference and 10 was the worst possible pain or the greatest sleep interference. The NRS and SIR were assessed on the morning of the 1st, 3rd and 5th day after surgery. Each patient was visited by a trained questioner who was blinded to the treatment groups and instructed to fill out the evaluation form.
In the event of severe pain, an analgesic drug including 50 mg diclofenac in a suppository form or 15 mg pentazocine in an intramuscular injection form was administered. Moreover, use of an intravenous PCV (iv-PCA) with fentanyl was possible as necessary according to an anesthesiologist’s decision. The number of additional analgesic drugs used in the first 5 days after surgery was recorded.
Adverse effects such as pneumonia, dysuria, headache, dizziness, nausea, nerve damage, stomach ache, peripheral edema, dry mouth, and constipation were evaluated for 5 days after surgery.
Operating room time indicates the time in the operation room. Anesthetic induction time indicates the time required after entering the operation room until the start of operation, including administration of epidural analgesia and also the time required for position change. Operation time indicates the time between the incision and closure. Recovery time indicates the time waking from anesthesia until leaving the operation room.
This study was a prospective controlled trial to investigate if the analgesic effect of pregabalin is significantly greater than that observed in epidural analgesia after thoracotomy. According to our previous study where the analgesic effects of NSAIDs and pregabalin against intercostal neuralgia were investigated, NRS values of the epidural analgesia group and the pregabalin group were determined to be 5.2 and 3.7, respectively, with a standard deviation of 2.0 (24,26). When these NRS values were analyzed using Student’s t-test, considering a significance level and detection power of 5% and 90%, respectively, with a 10% dropout rate, it was determined that a total of 96 patients were required for this study.
The patient characteristic data was analyzed with one-way ANOVA for continuous variables and χ2 test for categorical variables. The NRS pain scores were analyzed with Mann-Whitney U-test. SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) software package was used for statistical analyses. A value of P<0.05 was considered to be significant.
Results
A total of 138 patients were scheduled to undergo thoracotomy within 15 months beginning February 2014; 98 patients out of the 138, who were considered to be suitable for the study, were enrolled in this randomized study. Among those 96 patients, a total of 6 patients were withdrawn from the study at follow-up due to a change in operation procedure (2 patients), cancellation of surgery (2 patients), rejection of the consent (1 patient), and violation of protocol (1 patient). Therefore, the study was conducted on 90 patients, 45 in the epidural analgesia group and 45 in the pregabalin group (Figure 1).
There were no substantial differences among the groups with regard to age, gender, diagnosis, location of incision, or type of resection (Table 1).
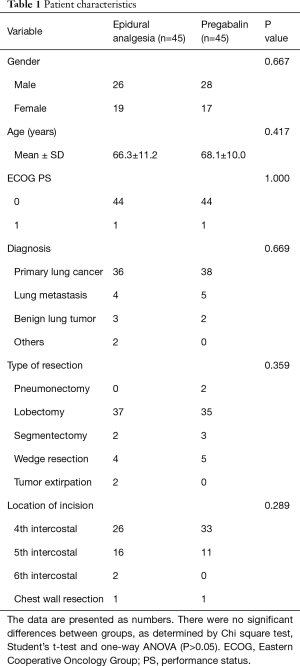
Full table
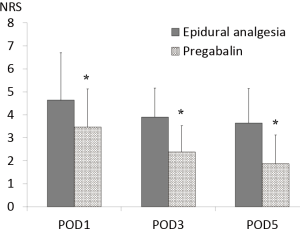
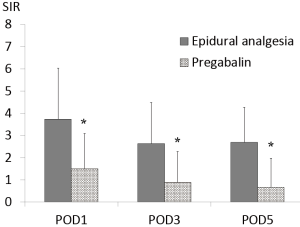
NRS and SIR were significantly lower in the pregabalin group compared with those in the epidural analgesia group over the entire period examined (NRS; POD1 4.64±2.05 vs. 3.47±1.65, P=0.006, POD3 3.89±1.27 vs. 2.38±1.15, P<0.001, POD5 3.61±1.50 vs. 1.87±1.25, P<0.001) (SIR; POD1 3.73±2.30 vs. 1.51±1.58, P<0.001, POD3 2.64±1.85 vs. 0.89±1.39, P<0.001, POD5 2.69±1.58 vs. 0.67±1.31, P<0.001) (Figures 2,3).
An ANOVA analysis demonstrated that pregabalin decreased the need for additional analgesic drugs for pain (P<0.05). With the exception of the day following surgery (P=0.678), all days from day 1 to 5 showed a significant effect of pregabalin, in decreasing the need for rescue (Table 2). The greatest effects were observed on days 1, 2 and 3, however days 4 and 5 also showed significant decrease. The additional analgesic drug administered for pain was mainly a 50-mg diclofenac suppository. No iv-PCA was administered.
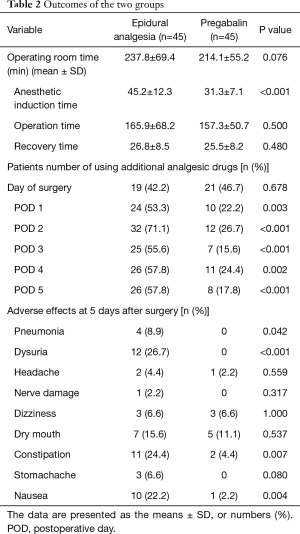
Full table
No difference was found in operating room time between the epidural analgesia group and pregabalin group (237.8±69.4, 214.1±55.2 min, P=0.076) while the epidural analgesia group showed a significantly longer anesthetic induction time compared with that in the pregabalin group (45.2±12.3, 31.3±7.1 min, P<0.001). There were no differences in operation time and recovery time between the two groups (P=0.500, P=0.480) (Table 2).
Adverse effects observed in the epidural analgesia group and the pregabalin group were pneumonia in 4 patients and 0 patients (P=0.042), dysuria in 12 patients and 0 patients (P<0.001), constipation in 11 patients and 2 patients (P=0.007), and nausea in 10 patients and 1 patient (P=0.004), respectively. Although dizziness has been reported among pregabalin users, the epidural analgesia group and the pregabalin group did not show a significant difference in the number of patients who developed dizziness, where both groups showed 3 patients (P=1.000) (Table 2).
Discussion
In this prospective and randomized controlled study to compare epidural analgesia and pregabalin for post-thoracotomy pain, pregabalin was found to be superior to epidural analgesia for treating acute phases of post-thoracotomy pain.
Post-thoracotomy pain occurs at a frequency of 20–80% (1-9). Various studies have been conducted with pain treatments such as intercostal nerve block, iv-PCA, and ketamine administration (5,7,27,28). However, the optimum method to treat post-thoracotomy pain is still controversial. It is known that NSAIDs are effective for treating nociceptive pain after surgery (9,24,26). However, since NSAIDs alone are not sufficient for treating post-thoracotomy pain, treatment for neuropathic pain is also necessary (24).
Thoracic epidural analgesia is considered to be the gold standard for perioperative pain management for thoracotomy. Epidural analgesia is thought to be effective for acute and chronic post-thoracotomy pain (10-12). However, this is still controversial. Moreover, serious complications such as nerve damage, epidural hematoma, epidural abscess, intrathecal or intravenous migration of the catheter have been reported in 0.2% of patients who received epidural analgesia (15-18). Epidural analgesia is not suitable for patients who are receiving antithrombotic therapy for preventing epidural hematoma (17). With the increase in an aging population, the number of patients undergoing antithrombotic therapy will increase as well. Moreover, since long term use of epidural analgesia can cause epidural abscess, pain management after catheter removal can become an issue (18). The development of an innovative, simple, safe and precise treatment method is envisaged.
Pregabalin is a drug which binds to Ca2+ channels in nerve presynapses, suppressing the release of neurotransmitters and resulting in an analgesic effect (19-23). There have been many reports showing excellent efficacy of pregabalin against neuropathic pain and postoperative pain (29-32). Our group has reported the effectiveness of pregabalin for treating chronic post-thoracotomy pain (24-26). On the other hand, Brulotte et al. recently reported that pregabalin did not suppress the incidence of post-thoracotomy pain occurrence (33). However, he indicated in the report that pregabalin was effective in reducing the severity of post-thoracotomy pain, resulting in lower additional analgesic drug use.
Post-thoracotomy pain affects patient quality of life which can lead to sleep disorders (25,34). This study revealed that SIR after surgery was significantly lower in the pregabalin group. This is thought to be an effect of pain relief, and partly due to side effects of pregabalin, which induces drowsiness.
Induction of epidural analgesia requires expertise as well as an extended period of time. Anesthetic induction time of the epidural analgesia group was approximately 15 min longer than that of the pregabalin group. In this study, the amount of anesthesia used was not defined and was determined by an anesthesiologist. However, both operation time and recovery time were not influenced by the amount of anesthesia.
No difference was observed between the two groups in the number of patients who required additional analgesic drugs on the day following surgery (42.2% vs. 46.7%, P=0.678). After POD1, 53.3–71.1% of patients in the epidural analgesia group required additional analgesic drugs compared to only 17.8–26.7% of patients in the pregabalin group (P<0.005). Therefore, it was found that oral administration of pregabalin was effective for post-thoracotomy pain.
Adverse effects observed among patients in the epidural analgesia group were pneumonia, urinary disorder, constipation, and nausea. Pneumonia, which may occur in patients after thoracotomy, can be caused by atelectasis, which may be due to the suppression of cough to avoid pain and leading to the accumulation of sputum in the lungs (35,36). Therefore, it is thought that pain is indirectly involved in the development of pneumonia among patients in the epidural analgesia group. Urinary retention, a complication associated with epidural analgesia, can be observed occasionally (37). In our hospital, patients who underwent thoracotomy started walking the day after surgery following urinary catheter removal. However, 12 patients in the epidural analgesia group (26.7%) required reattachment of a urinary catheter due to dysuria, where 8 were male (8/26, 30.8%) and 4 were female (4/19, 21.1%). Thus, there was no difference in gender among those receiving a urinary catheter. There have been reports of dizziness and sedation as complications associated with pregabalin; however, there was no difference in the number of occurrences of dizziness between the epidural analgesia group and the pregabalin group in this study.
Epidural analgesia reduces the use of additional analgesic drugs on the day following surgery, as well as lowering NRS below 4.6 after surgery. The effectiveness of epidural analgesia against post-thoracotomy was shown here. However, the use of pregabalin can be an effective alternative analgesic drug which may be administered to patients who have difficulty using epidural analgesia or when epidural analgesia is not effective. It is believed that the post-operative use of multimodal analgesia, which includes epidural analgesia, pregabalin and NSAIDs, will have the greatest analgesic benefit.
There are three study limitations. One limitation is that bias may occur on how to evaluate pain due to the open study. Therefore, it is necessary to conduct a double-blind study using a placebo. A second limitation is that there may be an effect of anesthesia on post-thoracotomy pain immediately after surgery since the amount of anesthesia used during surgery was not defined. A third limitation is that this study was conducted at a single facility. Therefore, this study should be conducted at multiple facilities to generalize results.
Conclusions
Pregabalin is a safe and effective analgesic drug which may be an alternative to epidural analgesia to treat acute post-thoracotomy pain.
Acknowledgements
This work was supported by Pfizer Japan Inc., Noriyuki Matsutani has received a research grant from Pfizer Japan Inc. [Pfizer Reference # WI184393]. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This prospective and randomized controlled study was approved by the Institutional Ethics Review Board (Teikyo University Review Board 13-122), and the study was registered on the University Hospital Medical Information Network (UMIN000013189). All participants provided written informed consent prior to the study.
References
- Williams EH, Williams CG, Rosson GD, et al. Neurectomy for treatment of intercostal neuralgia. Ann Thorac Surg 2008;85:1766-70. [Crossref] [PubMed]
- Rogers ML, Henderson L, Mahajan RP, et al. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg 2002;21:298-301. [Crossref] [PubMed]
- Steegers MA, Snik DM, Verhagen AF, et al. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955-61. [Crossref] [PubMed]
- Mendola C, Cammarota G, Netto R, et al. S+ -ketamine for control of perioperative pain and prevention of post thoracotomy pain syndrome: a randomized, double-blind study. Minerva Anestesiol 2012;78:757-66. [PubMed]
- Tena B, Gomar C, Rios J. Perioperative epidural or intravenous ketamine does not improve the effectiveness of thoracic epidural analgesia for acute and chronic pain after thoracotomy. Clin J Pain 2014;30:490-500. [Crossref] [PubMed]
- Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand 1999;43:563-7. [Crossref] [PubMed]
- Ryu HG, Lee CJ, Kim YT, et al. Preemptive low-dose epidural ketamine for preventing chronic postthoracotomy pain: a prospective, double-blinded, randomized, clinical trial. Clin J Pain 2011;27:304-8. [Crossref] [PubMed]
- Grosen K, Laue Petersen G, Pfeiffer-Jensen M, et al. Persistent post-surgical pain following anterior thoracotomy for lung cancer: a cross-sectional study of prevalence, characteristics and interference with functioning. Eur J Cardiothorac Surg 2013;43:95-103. [Crossref] [PubMed]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. [Crossref] [PubMed]
- De Cosmo G, Aceto P, Gualtieri E, et al. Analgesia in thoracic surgery Minerva Anestesiol 2009;75:393-400. review. [PubMed]
- Gottschalk A, Cohen SP, Yang S, et al. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594-600. [Crossref] [PubMed]
- Ng A, Swanevelder J. Pain relief after thoracotomy: is epidural analgesia the optimal technique? Br J Anaesth 2007;98:159-62. [Crossref] [PubMed]
- Bong CL, Samuel M, Ng JM, et al. Effects of preemptive epidural analgesia on post-thoracotomy pain. J Cardiothorac Vasc Anesth 2005;19:786-93. [Crossref] [PubMed]
- Stoelben E, Ludwig C. Chest wall resection for lung cancer: indications and techniques. Eur J Cardiothorac Surg 2009;35:450-6. [Crossref] [PubMed]
- Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth 2001;87:47-61. [Crossref] [PubMed]
- Giebler RM, Scherer RU, Peters J. Incidence of neurologic complications related to thoracic epidural catheterization. Anesthesiology 1997;86:55-63. [Crossref] [PubMed]
- Scott DA, Beilby DS, McClymont C. Postoperative analgesia using epidural infusions of fentanyl with bupivacaine. A prospective analysis of 1,014 patients. Anesthesiology 1995;83:727-37. [Crossref] [PubMed]
- Phillips JM, Stedeford JC, Hartsilver E, et al. Epidural abscess complicating insertion of epidural catheters. Br J Anaesth 2002;89:778-82. [Crossref] [PubMed]
- Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003;60:1274-83. [Crossref] [PubMed]
- Sabatowski R, Gálvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004;109:26-35. [Crossref] [PubMed]
- Tölle T, Freynhagen R, Versavel M, et al. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain 2008;12:203-13. [Crossref] [PubMed]
- Clarke H, Bonin RP, Orser BA, et al. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012;115:428-42. [Crossref] [PubMed]
- Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015;114:10-31. [Crossref] [PubMed]
- Matsutani N, Dejima H, Takahashi Y, et al. Pregabalin reduces post-surgical pain after thoracotomy: a prospective, randomized, controlled trial. Surg Today 2015;45:1411-6. [Crossref] [PubMed]
- Matsutani N, Kawamura M. Significant improvement of chronic pain by Pregabalin after thoracotomy: report of four cases. Surg Today 2013;43:915-7. [Crossref] [PubMed]
- Matsutani N, Kawamura M. Successful management of postoperative pain with pregabalin after thoracotomy. Surg Today 2014;44:712-5. [Crossref] [PubMed]
- Perttunen K, Nilsson E, Heinonen J, et al. Extradural, paravertebral and intercostal nerve blocks for post-thoracotomy pain. Br J Anaesth 1995;75:541-7. [Crossref] [PubMed]
- Concha M, Dagnino J, Cariaga M, et al. Analgesia after thoracotomy: epidural fentanyl/bupivacaine compared with intercostal nerve block plus intravenous morphine. J Cardiothorac Vasc Anesth 2004;18:322-6. [Crossref] [PubMed]
- Hill CM, Balkenohl M, Thomas DW, et al. Pregabalin in patients with postoperative dental pain. Eur J Pain 2001;5:119-24. [Crossref] [PubMed]
- Mathiesen O, Jacobsen LS, Holm HE, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth 2008;101:535-41. [Crossref] [PubMed]
- Buvanendran A, Kroin JS, Della Valle CJ, et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg 2010;110:199-207. [Crossref] [PubMed]
- Agarwal A, Gautam S, Gupta D, et al. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth 2008;101:700-4. [Crossref] [PubMed]
- Brulotte V, Ruel MM, Lafontaine E, et al. Impact of pregabalin on the occurrence of postthoracotomy pain syndrome: a randomized trial. Reg Anesth Pain Med 2015;40:262-9. [Crossref] [PubMed]
- Alar T, Ceylan KC, Kaya SO, et al. How does the type of thoracotomy affect the patient quality of life? A short form-36 health survey study. Surg Today 2014;44:264-70. [Crossref] [PubMed]
- Sabanathan S, Richardson J, Mearns AJ. Management of pain in thoracic surgery. Br J Hosp Med 1993;50:114-20. [PubMed]
- Amr YM, Yousef AA, Alzeftawy AE, et al. Effect of preincisional epidural fentanyl and bupivacaine on postthoracotomy pain and pulmonary function. Ann Thorac Surg 2010;89:381-5. [Crossref] [PubMed]
- Allen MS, Blackmon SH, Nichols FC 3rd, et al. Optimal Timing of Urinary Catheter Removal After Thoracic Operations: A Randomized Controlled Study. Ann Thorac Surg 2016;102:925-30. [Crossref] [PubMed]



