Airway stent complications: the role of follow-up bronchoscopy as a surveillance method
Introduction
Over the past two decades, airway stenting has become an integral part of the therapeutic endoscopic management of obstructive benign and malignant central airway diseases. Airway stent insertions often achieve immediate relief of dyspnea (1-6). Moreover, airway stenting has consistently improved airflow obstruction parameters on pulmonary function testing (3,7-10). Significant improvement in the Eastern Cooperative Oncology Group (ECOG) performance score has also been reported in patients with malignant disease after stent placement (2,11).
Along with well-established palliative and therapeutic benefits, stent-related complications have also been reported with use of metallic and silicone stents (2,5,12-17). These include stent migrations, stent fractures, stent-associated infections, and stent obstructions by tumor, granulation tissue and secretions. Follow-up endoscopic interventions are often needed to maintain airway patency and prevent further complications (6,12,17). Despite increased use of airway stents and frequent stent-associated complications, most interventional pulmonologists do not have defined follow-up or maintenance protocols to adhere to (18).
This study represents our institutional experience of various airway stent placements and follow-up bronchoscopies for management of obstructive benign and malignant central airway diseases. The study hypothesis is that follow-up bronchoscopy 4-6 weeks after stent placement allows for early detection of stent-related complications regardless of symptomatic status, which may serve as an optimal surveillance point to prevent further respiratory complications.
Methods
Study design
We performed a retrospective cohort study of all patients who underwent placement of at least one airway stent at the Johns Hopkins Hospital from April 2010 to December 2013. Patients were identified using the following current procedural terminology (CPT) codes: 31631 [bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with placement of tracheal stent(s)], 31636 [bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with placement of bronchial stent(s), initial bronchus], and 31637 [bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; each additional major bronchus stented].
The study was approved by the Johns Hopkins institutional review board (00028714) with waiver to informed consent provided. Patient data was retrieved from electronic medical records using a standardized form. All definitions were developed prior to chart abstraction to ensure consistency. Demographic (age, gender), stent data (stent type, location, and indication) and surveillance bronchoscopy findings were collected.
Follow-up bronchoscopy was performed according to the clinical protocol of our department for airway stenting, between 4–6 weeks following stent insertion or if patients became symptomatic, whichever occurred earlier. For patients who underwent a follow-up bronchoscopy any time after the initial stent insertion, we abstracted data on the type of bronchoscope used, symptomatic status prior to the bronchoscopy, stent-related complications, and therapeutic interventions performed during bronchoscopy. The primary outcome was development of stent-related complications, defined as stent migrations/fractures or obstruction by tumor, granulation tissue or secretions detected on follow-up bronchoscopy.
Statistical analysis
Patient and stent characteristics are presented overall, and by follow-up bronchoscopy status as means (standard deviations) for continuous variables and as percentages for categorical variables. Characteristics by follow-up bronchoscopy status were compared using Student’s t-tests and chi square tests. Clustering of stents within patients who had more than one stent placed during initial bronchoscopy was accounted for using the generalized estimating equations (GEE) model with independent correlation structure. Odds ratios (ORs) (with 95% CIs) for the presence of complications among those who underwent follow-up bronchoscopy were obtained from the GEE models. Univariate and multivariate analyses were performed. Variables included in the models were: age, sex, indication for stent placement, stent location, stent type and symptoms at the time of follow-up bronchoscopy. All reported P values are two-sided and P<0.05 was considered statistically significant. All analyses were performed using Stata Version 13 (StataCorp, College Station, Texas, USA).
Results
Patient characteristics
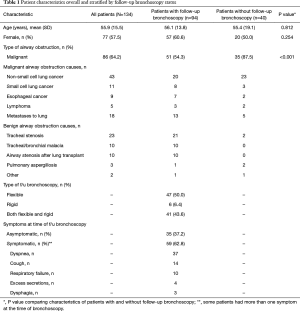
There were 134 patients with central airway obstruction with a total of 147 airway stents placed. Ten patients had more than one stent placed during the initial bronchoscopy. Seventy seven patients (58%) were women (Table 1). Mean patient age was 55.9±15.5 years. Eighty six patients (64%) underwent stent placement for malignant airway disease, half of whom had non-small cell lung cancer, while other malignant indications included small cell lung cancer, esophageal cancer, lymphoma, and lung metastases from variety of primary malignancies (6 sarcoma, 3 breast, 2 renal, 2 thyroid, 1 head and neck, 1 hepatoblastoma and 1 unknown primary).
Full table
Leading benign indications for airway stenting (48 patients) were idiopathic or post-intubation tracheal stenosis (23 patients), as well as post lung transplantation bronchial stenosis, tracheobronchomalacia, pulmonary aspergillosis, papillomatosis and tracheoesophageal fistula.
Among the 134 patients, who had an airway stent placed during the initial bronchoscopy, 94 (70%) had follow-up bronchoscopy of which 61% were women, 54% had a malignant indication for stent placement, and 63% were symptomatic at the time of the bronchoscopy (Table 1). These 94 patients had a total of 100 stents (5 patients had more than one stent). The mean time from stent placement to follow-up bronchoscopy was 41.2 days. There were no complications associated with follow-up bronchoscopy.
Stent characteristics
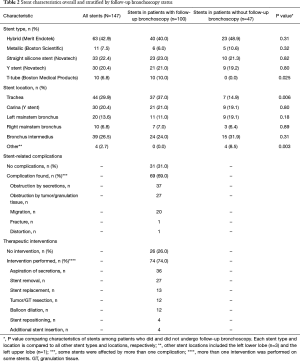
The types and locations of the stents inserted during the initial bronchoscopy are outlined in Table 2. The most common stent was the hybrid stent (42.9%), followed by the straight silicone stent (22.4%), and the Y-stent (20.4%). The metallic stent accounted for only 7.5% of the stents, as it was taken off the market in 2011. All metallic stent placements were performed for malignant airway obstruction only. Of note, all patients with a T-tube had a follow-up bronchoscopy. Tracheal stents placed for indications of tracheal stenosis and tracheomalacia were well represented in the group of patients who underwent follow-up bronchoscopy.
Full table
More than two thirds of all stents placed were associated with at least one complication at the time of the follow-up bronchoscopy, such as migration, fracture, and obstruction by tumor, granulation tissue and secretions (Table 2). Nineteen stents were found to have complications within 5 days of placement, requiring therapeutic intervention during the follow-up bronchoscopy.
Analysis of stent complications
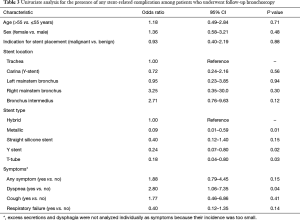
In univariate analysis of patients, who underwent follow-up bronchoscopy, the presence of dyspnea and having hybrid stents were independent predictive factors of stent-related complications (Table 3). Stent-related complications did not necessarily correlate with respiratory symptoms [odds ratio (OR) 1.88, 95% CI: 0.79–4.45], as 60% of asymptomatic patients were found to have at least one stent-related complication on follow-up bronchoscopy. Moreover, patient age, sex, and stent indication/location, were not predictive of development of stent-related complications.
Full table
In multivariate analysis, the presence of dyspnea was no longer an independent predictive factor of stent-related complications discovered during the follow-up bronchoscopy (OR 2.36, 95% CI: 0.72–7.70). The hybrid stents continued to have increased risk of stent-related complications compared to the metallic, Y, and T-tube stents (metallic: OR 0.02, 95% CI: 0.002–0.29; Y-stent: OR 0.10, 95% CI: 0.01–0.78; T-tube: OR 0.06, 95% CI: 0.01–0.60). Of note, the straight silicone stent was associated with lower odds of complications compared to the hybrid stent, although this association did not reach statistical significance (OR 0.17, 95% CI: 0.03–1.00).
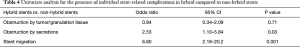
Given the higher incidence of stent-related complications with hybrid stents, we performed further analysis to determine the likelihood of individual stent-related complications associated with hybrid stents, compared to all other stents used in our study. Stent migration (OR =6.60, 95% CI: 2.16–20.2) and stent obstruction by secretions (OR =2.53, 95% CI: 1.10–5.84) were significantly more likely to occur with hybrid stents (Table 4).
Full table
Discussion
Approximately 30% of patients with lung cancer develop central airway obstruction and may require airway stents as part of their management (19). Though surgical resection remains the gold standard for management of benign airway stenosis, airway stents can serve as a trial to assess for symptomatic improvement prior to surgery and also can be therapeutically utilized in patients with inoperable strictures or deemed poor surgical candidates due to underlying comorbidities (20).
For both malignant and benign airway diseases, airway stents provide significant symptomatic benefit, decrease airflow obstruction, and improve quality of life (1-11). Despite many benefits, airway stents are foreign bodies prone to complications (21). Even with frequent stent-associated complications, guidelines for surveillance and maintenance of these stent remain inadequate18. This study confirms that stent-related complications are common, and highlights the potential role of follow-up bronchoscopy as a stent surveillance method.
The reported stent-related complication rate has varied in the literature (mostly 40–60%, and as high as 87% at 20 months), based on the study population and the types/material of airway stents deployed (2,13-17,22). The complication rate in our study (69%) falls within this range.
Stent complications have often been shown to occur within the first 2 to 3 months following stent placement and some, such as stent migration and granulation tissue formation, may manifest as early as 3 days later (4,12,23,24). In fact, nineteen stents (19.0%) in our study were found to have complications within 5 days of insertion.
In addition, loss of airway stent patency is most rapid during the first year following stent placement, often requiring bronchoscopic interventions (6,12,15). For metallic stents, neoepithelialization with incorporation of the stent within the airway wall can occur as early as 3–6 weeks; complications during removal of these stents were more likely to occur the longer they had been in place (25,26).
In benign airway stenosis, temporary airway stents can potentially provide long-term airway patency even after the stent removal. Appropriate surveillance and maintenance of these stents is essential, as longer these stents are kept, the higher the likelihood of long-term airway patency after stent removal (27). These findings from our and other studies suggest that stent-related complications are common, and can occur early, and often require early therapeutic intervention to maintain airway patency.
While presence of symptoms in patients after stent placement is a clear indication for a follow-up bronchoscopy, the method of stent surveillance in asymptomatic patients is less clear and unestablished. In one study, routine surveillance bronchoscopy of asymptomatic patients within 2 to 3 months after stent insertion did not result in detection of high incidence of stent-related complications (28). In our study, however, absence of symptoms did not always correlate with stent-associated complications. The two studies are similar in size and patient population, but our study examined multiple stent types (metallic, silicone, and hybrid), rather than silicone stents alone, which may explain the difference in findings. Our study suggests that routine surveillance bronchoscopy, regardless of symptomatic status, may play a role in early detection of stent-related complications, with a goal to delay likely future sequelae of post-obstructive pneumonia and respiratory failure. The impact of routine surveillance bronchoscopy on patient mortality, morbidity, hospitalization, and overall health costs remains to be determined.
A survey by Hoag et al. revealed that 61% of interventional pulmonologists discovered stent-related complications via various surveillance methods, including physical examination, chest radiography, computed tomography (CT), bronchoscopy and fluoroscopy (18). There is limited data to compare the efficacy of these different surveillance methods at detecting stent-related complications. Small studies showed that CT was an accurate, non-invasive method to evaluate airway stents and airway patency, though unable to characterize stent epithelialization and subtle stenoses (29,30).
To our knowledge, our study includes the largest number of hybrid stents ever studied. The hybrid stent is a self-expanding stent, which combines the features of metallic and silicone stents, with a hydrophilic coating and completely covered nitinol framework. Our study shows that hybrid stents are associated with higher rates of migration and significant obstruction by secretions, compared to other stents. Smaller studies have also reported the occurrence of these complications. In one study, which evaluated recurrent bronchus intermedius strictures in lung transplant recipients, mucus buildup was detected in all 6 stents, and immediate migration was seen in 5 out of 6 stents (31). In another study, four out of five hybrid stents were affected by migration in benign airway stenoses (13).
The strengths of our study include the relatively large patient sample, the diversity of indications for stent placement, and the variety of stents used. The main limitation of our study was its retrospective nature; as such, multicenter, prospective studies are needed to validate the optimal period of surveillance bronchoscopy for long-term benefits. Another limitation of our study was that about a third of our patients did not undergo follow-up bronchoscopy due to primarily to death or transition to hospice care. Furthermore, we did not evaluate for stent-associated respiratory tract infection, which is known to occur in 15 to 20% of patients with stents (16,32,33). Finally, it remains to be determined, whether the early bronchoscopic detection of stent-related complications in asymptomatic patients can prevent subsequent symptom development or clinical deterioration. Prospective trials are needed to adequately define the role of surveillance bronchoscopy.
Conclusions
Surveillance bronchoscopy within 4 to 6 weeks of stent placement may be useful for early detection of complications and their subsequent management, regardless of symptomatic status and indication for stent placement. Prospective multicenter studies are needed to compare optimal surveillance methods and determine how surveillance bronchoscopy affects patient mortality, morbidity and healthcare costs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
Ethical Statement: The study was approved by the Johns Hopkins institutional review board (00028714) with waiver to informed consent provided.
References
- Dutau H, Toutblanc B, Lamb C, et al. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest 2004;126:951-8. [Crossref] [PubMed]
- Furukawa K, Ishida J, Yamaguchi G, et al. The role of airway stent placement in the management of tracheobronchial stenosis caused by inoperable advanced lung cancer. Surg Today 2010;40:315-20. [Crossref] [PubMed]
- Gelb AF, Zamel N, Colchen A, et al. Physiologic studies of tracheobronchial stents in airway obstruction. Am Rev Respir Dis. 1992;146:1088-90. [Crossref] [PubMed]
- Inchingolo R, Sabharwal T, Spiliopoulos S, et al. Tracheobronchial stenting for malignant airway disease: long-term outcomes from a single-center study. Am J Hosp Palliat Care 2013;30:683-9. [Crossref] [PubMed]
- Kim J, Shin JH, Kim JH, et al. Metallic stent placement for the management of tracheal carina strictures and fistulas: technical and clinical outcomes. AJR Am J Roentgenol 2014;202:880-5. [Crossref] [PubMed]
- Thornton RH, Gordon RL, Kerlan RK, et al. Outcomes of tracheobronchial stent placement for benign disease. Radiology 2006;240:273-82. [Crossref] [PubMed]
- Eisner MD, Gordon RL, Webb WR, et al. Pulmonary function improves after expandable metal stent placement for benign airway obstruction. Chest 1999;115:1006-11. [Crossref] [PubMed]
- Gotway MB, Golden JA, LaBerge JM, et al. Benign tracheobronchial stenoses: changes in short-term and long-term pulmonary function testing after expandable metallic stent placement. J Comput Assist Tomogr 2002;26:564-72. [Crossref] [PubMed]
- Hauck RW, Römer W, Schulz C, et al. Ventilation perfusion scintigraphy and lung function testing to assess metal stent efficacy. J Nucl Med 1997;38:1584-9. [PubMed]
- Vergnon JM, Costes F, Bayon MC, et al. Efficacy of tracheal and bronchial stent placement on respiratory functional tests. Chest 1995;107:741-6. [Crossref] [PubMed]
- Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088-93. [Crossref] [PubMed]
- Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg 2011;6:46. [Crossref] [PubMed]
- Dooms C, De Keukeleire T, Janssens A, et al. Performance of fully covered self-expanding metallic stents in benign airway strictures. Respiration 2009;77:420-6. [Crossref] [PubMed]
- Gildea TR, Murthy SC, Sahoo D, et al. Performance of a self-expanding silicone stent in palliation of benign airway conditions. Chest 2006;130:1419-23. [Crossref] [PubMed]
- Madden BP, Loke TK, Sheth AC. Do expandable metallic airway stents have a role in the management of patients with benign tracheobronchial disease? Ann Thorac Surg 2006;82:274-8. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, Mehta AC. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72; discussion 173-4. [Crossref] [PubMed]
- Hoag JB, Sherman M, Lund ME. Practice patterns for maintaining airway stents deployed for malignant central airway obstruction. J Bronchology Interv Pulmonol 2010;17:131-5. [Crossref] [PubMed]
- Stöhr S, Bolliger CT. Stents in the management of malignant airway obstruction. Monaldi Arch Chest Dis 1999;54:264-8. [PubMed]
- Grillo HC, Donahue DM. Postintubation tracheal stenosis. Chest Surg Clin N Am 1996;6:725-31. [PubMed]
- Zakaluzny SA, Lane JD, Mair EA. Complications of tracheobronchial airway stents. Otolaryngol Head Neck Surg 2003;128:478-88. [Crossref] [PubMed]
- Eller RL, Livingston WJ 3rd, Morgan CE, et al. Expandable tracheal stenting for benign disease: worth the complications? Ann Otol Rhinol Laryngol 2006;115:247-52. [Crossref] [PubMed]
- Cosano Povedano A, Muñoz Cabrera L, et al. Endoscopic treatment of central airway stenosis: five years' experience. Arch Bronconeumol 2005;41:322-7. [PubMed]
- Gaissert HA, Grillo HC, Wright CD, et al. Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg 2003;126:744-7. [Crossref] [PubMed]
- Alazemi S, Lunn W, Majid A, et al. Outcomes, health-care resources use, and costs of endoscopic removal of metallic airway stents. Chest 2010;138:350-6. [Crossref] [PubMed]
- Lunn W, Feller-Kopman D, Wahidi M, et al. Endoscopic removal of metallic airway stents. Chest 2005;127:2106-12. [Crossref] [PubMed]
- Kim JH, Shin JH, Song HY, et al. Benign tracheobronchial strictures: long-term results and factors affecting airway patency after temporary stent placement. AJR Am J Roentgenol 2007;188:1033-8. [Crossref] [PubMed]
- Matsuo T, Colt HG. Evidence against routine scheduling of surveillance bronchoscopy after stent insertion. Chest 2000;118:1455-9. [Crossref] [PubMed]
- Ferretti GR, Knoplioch J, Bricault I, et al. Central airway stenoses: preliminary results of spiral-CT-generated virtual bronchoscopy simulations in 29 patients. Eur Radiol 1997;7:854-9. [Crossref] [PubMed]
- Ferretti GR, Kocier M, Calaque O, et al. Follow-up after stent insertion in the tracheobronchial tree: role of helical computed tomography in comparison with fiberoptic bronchoscopy. Eur Radiol 2003;13:1172-8. [PubMed]
- Tan JH, Fidelman N, Durack JC, et al. Management of recurrent airway strictures in lung transplant recipients using AERO covered stents. J Vasc Interv Radiol 2010;21:1900-4. [Crossref] [PubMed]
- Agrafiotis M, Siempos II, Falagas ME. Infections related to airway stenting: a systematic review. Respiration 2009;78:69-74. [Crossref] [PubMed]
- Grosu HB, Eapen GA, Morice RC, et al. Stents are associated with increased risk of respiratory infections in patients undergoing airway interventions for malignant airways disease. Chest 2013;144:441-9. [Crossref] [PubMed]


